Abstract
Background
Introduction of online adaptive MR-guided radiotherapy enables stereotactic body radiation therapy (SBRT) of upper abdominal tumors. This study aimed to evaluate the feasibility of MR-guided SBRT on a 1.5 T MR-linac in patients with unresectable upper abdominal malignancies.
Material and methods
Patients treated at the UMC Utrecht (April 2019 to December 2020) were identified in the prospective ‘Multi-OutcoMe EvaluatioN of radiation Therapy Using the MR-linac’ (MOMENTUM) study. Feasibility of treatment was arbitrarily defined as an on-table time interval of ≤60 min for >75% of delivered fractions and completion of >95% of fractions as scheduled, reflecting patient tolerability. Acute treatment-related toxicity was assessed at 3 months of follow-up and graded according to the National Cancer Institute Common Terminology Criteria of Adverse Events version 5.0.
Results
Twenty-five consecutive patients with a median follow-up time of 8 (range 4–23) months were treated with 35 Gray (n = 4) and 40 Gray (n = 21) in five fractions over 2 weeks. For all fractions, contours were adapted based on the daily anatomy and delivered within 47 min/fraction (range 30–74). In 98/117 fractions (84%), adapted treatment was completed within 1 h. All patients received the scheduled irradiation dose as planned. No acute grade 3 toxicity or higher was reported. Treatment resulted in pain alleviation in 11/13 patients.
Discussion
Online adaptive MR-guided SBRT on a 1.5 T MR-linac is feasible and well-tolerated in patients with unresectable upper abdominal malignancies. Dose escalation studies, followed by comparative studies, are needed to determine the optimal radiation dose for irradiation of upper abdominal malignancies.
Introduction
In the therapeutic framework of malignancies in the upper abdomen, radiotherapy has played a minor role until recent years [Citation1–3]. Irradiation of upper abdominal tumors is restricted by adjacent surrounding organs with limited radiation tolerance, such as the duodenum, small bowel, and stomach: the organs at risk (OAR) [Citation4]. In addition, abdominal motion, due to respiration and peristalsis, necessitates the use of relatively large treatment volumes thereby limiting the deployment of dose escalation strategies [Citation5]. These factors have impeded the delivery of sufficient irradiation doses to control malignancies in the upper abdomen with conventional techniques [Citation6].
The development of fractionated, image-guided stereotactic body radiotherapy (SBRT) has allowed for reduced margins and an increase in radiation dose to lesions in the upper abdomen, without increasing the risk of injury to normal adjacent organs [Citation1,Citation7–10]. More recently, the introduction of MR-linac systems has further improved the applicability of SBRT for upper abdominal tumors [Citation11,Citation12]. These systems combine a linear accelerator and MRI scanner and enable online adaptive MR-guided radiotherapy. In contrast to conventional CT-guided radiotherapy, MR-guided online adaptive radiotherapy allows for the daily adaptation of the treatment plan based on the actual anatomy [Citation13,Citation14]. This enables high-precision radiotherapy potentially increasing irradiation doses to target lesions while sparing surrounding healthy tissues [Citation15,Citation16].
The theoretical advantages of MR-guided SBRT considerably impact the role of radiotherapy for malignancies in the upper abdomen and merit further investigation. The aim of this study was to evaluate the feasibility of online adaptive MR-guided radiotherapy on a 1.5 tesla (T) MR-linac in the first group of patients with unresectable upper abdominal malignancies.
Material and methods
This study complied with the R-IDEAL framework for the first clinical evaluation of MR-guided SBRT using the MR-linac (stage 1) and optimization of the online adaptive workflow (stage 2a) in patients with upper abdominal malignancies [Citation17].
Study population
All patients with unresectable, pathology-proven upper abdominal malignancies in the pancreatic or periampullary region who were treated with online adaptive MR-guided radiotherapy on a 1.5 T MR-linac (Elekta Unity, Elekta AB, Stockholm, Sweden) between April 2019 and December 2020 in the department of radiation oncology at the UMC Utrecht were included. Indications for MR-guided SBRT were determined by consensus during a multidisciplinary team meeting. Informed consent for the prospective Multi-OutcoMe EvaluatioN of radiation Therapy Using the MR-linac (MOMENTUM) study was provided, which has been approved by the institutional review board (NCT04075305) [Citation18].
Treatment planning and patient position
A dose of 35 Gray (Gy) was administered in five fractions to all patients who were treated before June 2019. Hereafter, the dose regimen was increased to 40 Gy in five fractions, based on initial experiences and national consensus. Fractions were spread over a period of 2 weeks, with at least 1 day between each fraction. For pretreatment imaging, a planning CT scan (Philips, Brilliance Big Bore CT) and MR-sim (1.5 T Philips ingenia MR-RT) of the entire upper abdomen was performed. The planning-CT protocol consisted of a four-dimensional (4 D) CT and an intravenous contrast-enhanced CT with an arterial and a portal venous phase, with a slice thickness of 3 mm and voxel size of 1.37 × 1.37 × 3 mm. For MRI, a 3 D T2 weighted (T2w) scan, dynamic T1 weighted sequential with and without intravenous contrast, and diffusion-weighted imaging (DWI) were acquired. Patients were positioned on a vacuum mattress (BlueBAG, Elekta AB, Stockholm, Sweden) in a head-first supine position with the arms along the body. A custom-made abdominal plaster corset was used to reduce breathing-induced tumor motion [Citation19]. Patient setup was indexed to a special table overlay used for CT acquisition [Citation20]. Tattooed skin marks were placed on the patient for reproducibility of the desired treatment position during treatment delivery.
Delineation and target coverage
All pretreatment scans were registered to the planning CT scan based on the anatomy of the target area. The gross tumor volume (GTV) and OARs were delineated on the mid-ventilation phase (20%) of the 4 D CT with support of the rigidly co-registered contrast-enhanced CT and MRI sequences. Delineation was performed by a radiation oncologist specialized in hepato-pancreato-biliary tumors in accordance with consensus delineation guidelines [Citation21]. The planning target volume (PTV) was defined as the GTV expanded with a 3-mm margin. All OARs located up to 3 cm cranially and 3 cm caudally of the PTV were delineated. In the case of locally recurrent disease after pancreatoduodenectomy, the different anastomoses were delineated. Dose constraints were based on consensus guidelines [Citation22]. The reference intensity-modulated treatment plan used the Monaco treatment planning system (version 5.40.01, Elekta AB, Stockholm, Sweden) using a static beam configuration of typically 9–14 beams. In case the dose constraints to the OARs were going to be violated, target coverage was compromised to a minimal extent.
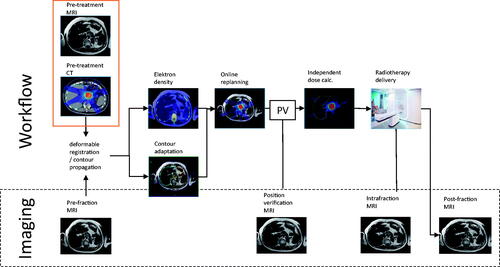
Treatment on the MR-linac
All patients were treated using an online adaptive workflow on the 1.5 T MR-linac (1.5 T MRI combined with 7 MV linear accelerator) [Citation23]. At the start of each fraction, a 3D T2w MRI treatment planning scan was acquired and fused with the planning CT scan based on the anatomy of the target area. The target and OAR delineations of the planning CT were non-rigidly propagated to this MRI using a deformable registration tool and manually adapted to comply with the actual anatomy. A full re-planning was performed and a new IMRT plan was generated using the same set of objectives and constraints as in the reference plan. In parallel, a 3D T2w MRI was acquired for position verification, to monitor anatomical changes between the end of the planning scan and the start of irradiation.
The steps of the workflow were timed by a radiotherapy technologist. The total on-table time per fraction was defined as the time between the patient leaving the dressing room until returning to the dressing room after the procedure. To prevent the formation of radiation-induced duodenum ulcers, a proton pump inhibitor was prescribed for a period of 6 months.
(Clinical) outcomes
Baseline clinical and tumor characteristics were prospectively obtained from MOMENTUM, as well as information on acute treatment-related toxicity and symptom palliation. Baseline clinical characteristics included sex, age, previous treatment, and presence of symptoms; tumor characteristics included tumor site, type of tumor, and tumor size defined as the GTV measured on pretreatment MRI. The pain was reported using a pain score between 0 and 10. Feasibility of treatment was arbitrarily scored as an on-table time interval of ≤60 min for >75% of delivered fractions and successful completion of >95% of fractions as scheduled, reflecting patient tolerability including treatment duration. Acute toxicity was defined as treatment-related toxicity occurring within 90 days after radiotherapy, graded according to the National Cancer Institute Common Terminology Criteria of Adverse Events (NCI CTCAE) version 5.0. Symptoms were evaluated at 3 months of follow-up to assess symptom alleviation.
Data analysis
Patient, tumor, and treatment characteristics, clinical symptoms and toxicity measurements were scored for each patient individually. Categorical parameters were counted as frequencies. Total on-table time per fraction, as well as the timings of the different steps of the workflow, was recorded as median (range). Data were analyzed using SPSS Version 25.0 (IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp).
Results
Study population
Twenty-five consecutive patients were treated, of whom five patients were diagnosed with a primary locally advanced pancreatic cancer, fifteen patients had an isolated local pancreatic or periampullary cancer recurrence, one patient had an isolated local recurrence of an adrenal gland carcinoma, two patients had a pancreatic metastasis of a renal cell carcinoma, one patient had a breast cancer metastasis in the pancreas, and one patient had a poorly differentiated malignancy in the upper abdomen with unclear origin (). The first four patients were irradiated to a dose of 35 Gy, and the other twenty-one patients received a dose of 40 Gy.
Table 1. Patient and tumor characteristics of 25 patients with upper abdominal malignancies treated with MR-guided SBRT on a 1.5 T MR-linac.
Treatment delivery
All patients completed treatment as planned, without any treatment breaks. A total of 117 online adapted fractions were delivered. The median on-table time was 47 min/fraction (range 30–74 min). In 98/117 fractions (84%), the overall adapted treatment time was less than 60 min. On-table time included re-contouring time by physician (median 13 min [range 3–38 min]), re-planning time (median 5 min [range 2–27 min]), and dose delivery time (median 11 min [range 1–32 min]) (). Delivery of fractions in more than 1 h (n = 19) was contributed to a learning curve regarding the online adaptation of contours since these 117 fractions were the first delivered on the MR-linac for abdominal tumors. Other reasons were the occurrence of time-consuming errors in the first period after implementation of the MR-linac and movement of a few patients within the MR-linac, requiring plan correction.
Clinical outcomes
Prior to the start of treatment, thirteen patients experienced abdominal pain or back pain, with a median pain score of 4 (range 1–9) (). Fatigue was present in three patients, and three patients experienced weight loss. During the first 3 months of follow-up after treatment, the patient reported grade 1–2 toxicity included fatigue (n = 11), diarrhea (n = 4), and nausea (n = 3). No acute grade 3 toxicity or higher was reported. Pain alleviation occurred in all but two patients during the first 3 months of follow-up.
Discussion
The results of this study show that online adaptive MR-guided SBRT is a feasible treatment option for patients with unresectable tumors in the upper abdomen. During each fraction, contours were adapted based on the anatomy of the day and delivered with a median on-table time of 47 min. In 84% of fractions, adapted treatment was completed within 1 h. All patients received the scheduled radiation dose without reporting serious toxicity.
It is widely recognized that local tumor growth of upper abdominal malignancies is associated with serious symptoms and significantly reduced quality of life [Citation24]. As advancements in systemic treatment have improved patient survival, effective therapies aiming to provide local tumor control and symptom alleviation are of increasing importance to enable survival with a good quality of life. To this purpose, the application of radiotherapy as a minimally invasive, local ablative therapy has gained interest in the treatment of various tumors in the upper abdomen [Citation25].
The potential to visualize tumors and adjacent tissues with MR-guided workflow techniques allow for a further increase in irradiation dose. As a result, the current study shows that high radiation doses can be safely delivered, while serious toxicity was not reported. Therefore, online adaptive MR-guided radiotherapy is a meaningful innovation for malignancies in the upper abdomen. This enables further investigation of the true value of radiotherapy for these tumor sites.
One study has been published, evaluating online adaptive MR-guided radiotherapy in twenty patients with liver and non-liver abdominal malignancies. In this phase I clinical trial, Henke et al. found that it was safe to apply 50 Gy in five fractions [Citation16]. As compared with other studies on SBRT in upper abdominal malignancies, a dose over 40 Gy is relatively high in this patient population [Citation3,Citation26–28]. Both in their study and in the current study, zero acute toxicity rates of grade 3 or higher occurred. Comparable to our study, in which plan adaptation was performed in all fractions, they adapted 88% of all fractions based on OAR constraints. In the study of Henke et al. this resulted in a median on-table time of 79 min/fraction, with 52% of all fractions being delivered in <80 min, and >75% in <90 min [Citation16]. As a result, the main outcome of feasibility in their study defined as completion of adaptive treatment <80 min for >75% of cases, was not met. Despite the fact that all fractions were adapted in the current study, delivery of adaptive treatment ≤80 min was achieved in 100% of fractions and ≤60 min in 84% of cases, with a median on-table time of 48 min/fraction. Only 19 of 117 fractions had a duration of longer than 60 min. Consequently, this study supports the conclusion of Henke et al. that online adaptive MRI-guided SBRT is safe in patients with upper abdominal malignancies, and additionally proves that SBRT is feasible in these patients.
In conclusion, this study shows encouraging results for further optimization of online adaptive MR-guided radiotherapy in patients with unresectable tumors in the upper abdomen. Plan adaptation based on the actual anatomy visualized on daily MRI images is feasible, well-tolerated and permits dose escalation to improve the efficacy of SBRT in patients with upper abdominal malignancies.
Disclosure statement
Dr. W.A. Hall receives institutional research and travel support from Elekta. Prof. H.M. Verkooijen receives research funding from Elekta.
Data availability statement
The data that support the findings of this study are available from the corresponding author, LD, upon reasonable request.
Additional information
Funding
References
- Ghaly M, Gogineni E, Saif MW. The evolving field of stereotactic body radiation therapy in pancreatic cancer. Pancreas. 2019;3(1):9–14.
- Henke L, Kashani R, Yang D, et al. Simulated online adaptive magnetic resonance-guided stereotactic body radiation therapy for the treatment of oligometastatic disease of the abdomen and central thorax: characterization of potential advantages. Int J Radiat Oncol Biol Phys. 2016;96(5):1078–1086.
- Hall WA, Straza MW, Chen X, et al. Initial clinical experience of stereotactic body radiation therapy (SBRT) for liver metastases, primary liver malignancy, and pancreatic cancer with 4D-MRI based online adaptation and real-time MRI monitoring using a 1.5 tesla MR-Linac. PLoS One. 2020;15(8):e0236570.
- Pollom EL, Chin AL, Diehn M, et al. Normal tissue constraints for abdominal and thoracic stereotactic body radiotherapy. Semin Radiat Oncol. 2017;27(3):197–208.
- Mostafaei F, Tai A, Omari E, et al. Variations of MRI-assessed peristaltic motions during radiation therapy. PLoS One. 2018;13(10):e0205917.
- Hammel P, Huguet F, van Laethem JL, et al. LAP07 trial group. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial. JAMA. 2016;315(17):1844–1853.
- Crane CH. Hypofractionated ablative radiotherapy for locally advanced pancreatic cancer. J Radiat Res. 2016;57(1):i53–i57.
- Zhong J, Patel K, Switchenko J, et al. Outcomes for patients with locally advanced pancreatic adenocarcinoma treated with stereotactic body radiation therapy versus conventionally fractionated radiation. Cancer. 2017;123(18):3486–3493.
- Park JJ, Hajj C, Reyngold M, et al. Stereotactic body radiation vs. intensity-modulated radiation for unresectable pancreatic cancer. Acta Oncol. 2017;56(12):1746–1753.
- Reyngold M, Parikh P, Crane CH. Ablative radiation therapy for locally advanced pancreatic cancer: techniques and results. Radiat Oncol. 2019;14(1):95.
- Rudra S, Jiang N, Rosenberg SA, et al. Using adaptive magnetic resonance image-guided radiation therapy for treatment of inoperable pancreatic cancer. Cancer Med. 2019;8(5):2123–2132.
- Hassanzadeh C, Rudra S, Bommireddy A, et al. Ablative five-fraction stereotactic body radiation therapy for inoperable pancreatic cancer using online MR-guided adaptation. Adv Radiat Oncol. 2021;6(1):100506.
- Raaymakers BW, Lagendijk JJ, Overweg J, et al. Integrating a 1.5 T MRI scanner with a 6 MV accelerator: proof of concept. Phys Med Biol. 2009;54(12):N229–N237.
- Lagendijk JJ, Raaymakers BW, van Vulpen M. The magnetic resonance imaging-linac system. Semin Radiat Oncol. 2014;24(3):207–209.
- Kontaxis C, Bol GH, Lagendijk JJ, et al. A new methodology for inter- and intrafraction plan adaptation for the MR-linac. Phys Med Biol. 2015;60(19):7485–7497.
- Henke L, Kashani R, Robinson C, et al. Phase I trial of stereotactic MR-guided online adaptive radiation therapy (SMART) for the treatment of oligometastatic or unresectable primary malignancies of the abdomen. Radiother Oncol. 2018;126(3):519–526.
- Verkooijen HM, Kerkmeijer LGW, Fuller CD, et al. R-IDEAL: a framework for systematic clinical evaluation of technical innovations in radiation oncology. Front Oncol. 2017;7:59.
- de Mol van Otterloo SR, Christodouleas JP, Blezer ELA, et al. The MOMENTUM study: an international registry for the evidence-based introduction of MR-guided adaptive therapy. Front Oncol. 2020;10(1328):1328–1329.
- Heerkens HD, Reerink O, Intven MPV, et al. Pancreatic tumor motion reduction by use of a custom abdominal corset. PhiRO. 2017;2:7–10.
- Werensteijn-Honingh AM, Kroon PS, Winkel D, et al. Feasibility of stereotactic radiotherapy using a 1.5 T MR-linac: multi-fraction treatment of pelvic lymph node oligometastases. Radiother Oncol. 2019;134:50–54.
- Heerkens HD, Hall WA, Li XA, et al. Recommendations for MRI-based contouring of gross tumor volume and organs at risk for radiation therapy of pancreatic cancer. Pract Radiat Oncol. 2017;7(2):126–136.
- Hanna GG, Murray L, Patel R, et al. UK consensus on normal tissue dose constraints for stereotactic radiotherapy. Clin Oncol. 2018;30(1):5–14.
- Winkel D, Bol GH, Kroon PS, et al. Adaptive radiotherapy: the elekta Unity MR-linac concept. Clin Transl Radiat Oncol. 2019;18:54–59.
- Cardillo N, Seible DM, Fero KE, et al. Clinical impact of local progression in pancreatic cancer. J Natl Compr Canc Netw. 2018;16(6):711–717.
- Wolny-Rokicka E, Sutkowski K, Grządziel A, et al. Tolerance and efficacy of palliative radiotherapy for advanced pancreatic cancer: a retrospective analysis of single-institutional experiences. Mol Clin Oncol. 2016;4(6):1088–1092.
- Mahadevan A, Jain S, Goldstein M, et al. Stereotactic body radiotherapy and gemcitabine for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2010;78(3):735–742.
- Chuong MD, Springett GM, Freilich JM, et al. Stereotactic body radiation therapy for locally advanced and borderline resectable pancreatic cancer is effective and well tolerated. Int J Radiat Oncol Biol Phys. 2013;86(3):516–522.
- Polistina F, Costantin G, Casamassima F, et al. Unresectable locally advanced pancreatic cancer: a multimodal treatment using neoadjuvant chemoradiotherapy (gemcitabine plus stereotactic radiosurgery) and subsequent surgical exploration. Ann Surg Oncol. 2010;17(8):2092–2101.