Abstract
Background
To report 5- and 7-year outcomes after image-guided moderately accelerated hypofractionated proton therapy (AHPT) for prostate cancer.
Material and methods
We reviewed the first 582 prostate cancer patients enrolled on prospective outcomes tracking trial and treated with double-scattered moderately AHPT between 2008 and 2015. 269 patients had low-risk (LR) and 313 had intermediate-risk (IR) disease, including 149 with favorable intermediate-risk (FIR) and 164 with unfavorable intermediate-risk (UIR) disease. LR patients received a median 70.0GyRBE (2.5GyRBE/fraction) and IR patients received a median of 72.5 GyRBE. Seventeen patients (UIR, n = 12) received androgen deprivation therapy (ADT) for a median of 6 months. Toxicities were graded per the CTCAE, v4.0, and patient-reported quality-of-life data were reviewed.
Results
Median follow-up was 8.0 years (0.9–12.2). The 5- and 7-year rates of freedom from biochemical progression (FFBP) overall and in the LR and IR subsets, respectively, were 96.8/95.2%, 98.8/98.8%, and 95.0/91.9%. For the FIR and UIR subsets, they were 97.2/95.2% and 93.1/88.8%. Actuarial 5- and 7-year rates of late CTCAE, v4.0, grade 2 gastrointestinal (GI), grade 3 GI, and grade 3 genitourinary (GU) toxicities were 9.9%/11.2%, 1.4/1.4% and 1.3/2.1%, respectively. No grade ≥4 GI or GU toxicities occurred. The mean (standard deviation, SD) IPSS and EPIC Composite bowel function and bother scores were 7 (SD = 5), 97 (SD = 7), and 94 (SD = 6), respectively at baseline, 7 (SD = 5), 92 (SD = 13), and 92 (SD = 9) at the 5-year follow-up, and 7 (SD = 5), 93 (SD = 12), and 92 (SD = 10) at the 7-year follow-up.
Conclusion
Image-guided AHPT 5- and 7-year outcomes show high efficacy, minimal physician-assessed toxicity, and excellent patient-reported outcomes in this cohort.
Background
Moderately accelerated hypofractionated (2.5–4 Gy/fraction) photon therapy is recognized in the American Society of Radiation Oncology (ASTRO), the American Society of Clinical Oncology (ASCO) and the American Urological Society (AUA) evidence-based guidelines as a standard-of-care treatment for prostate cancer [Citation1]. The shorter hypofractionated regimen has been demonstrated in prospective randomized trials to yield similar 5-year control rates and toxicities as conventionally fractionated (1.8–2.0 Gy/fraction) photon therapy, but with reduced resource utilization and cost [Citation2–5]. The ASTRO/ASCO/AUA guidelines have not included moderately accelerated hypofractionated proton therapy as a standard-of-care treatment for prostate cancer because mature data on clinical outcomes with this approach were limited and there is no level I evidence of non-inferiority compared with standard fractionated proton therapy. However, protons have dosimetric advantages over photons in reducing low- and moderate-dose radiation exposure to adjacent organs and published results have shown promising control rates and the potential to improve patient-reported outcomes of bowel function [Citation6,Citation7]. Comparison of intensity-modulated radiation therapy (IMRT) to proton therapy, with both conventionally fractionated and moderately accelerated hypofractionated regimes, is the subject of two ongoing national trials [Citation8,Citation9]. The purpose of this study is to report the 5- and 7-year outcomes of moderately AHPT in a large group of patients treated at the University of Florida Health Proton Beam Institute between 2008 and 2015.
Material and methods
The patients
This study includes 582 patients enrolled in an institutional review board (IRB)-approved outcomes tracking protocol between 2008 and 2015, most of whom were also enrolled on two successive prospective clinical trials described below. All 582 patients had biopsy-proven prostate cancer, clinical stage T1–T2b, prostate-specific antigen (PSA) level ≤20 ng/ml, Gleason Score ≤7, and no nodal or distant metastases. Of these, 215 patients were enrolled in an institutional review board-approved prospective trial of low-risk (LR) and intermediate-risk (IR) patients between April 2008 and October 2011. The 5-year outcomes in this cohort were previously reported [Citation10], and this group of patients was also included in a pooled toxicity analysis [Citation11]. Exclusion criteria on this initial study of hypofractionated proton therapy were as follows: prior prostate cancer surgery or local prostate cancer treatment; active inflammatory bowel disease affecting the rectum; history of proximal urethral stricture requiring dilatation; International Prostate Symptom Score (IPSS) of ≥15; use of alpha-blockers, diabetes mellitus; prior pelvic surgery; current and continuing use of anticoagulation or saw palmetto or immunosuppressants; and inability to meet prespecified dose constraints for organs at risk (OAR) in the treatment planning process. In addition, a protocol amendment excluded patients with a prostate volume of ≥60 cm3 after April 16, 2009. The use of androgen deprivation therapy (ADT) was discouraged. However, 2 patients had a single 3-month injection of gonadotropin-releasing hormone agonist before proton therapy, 1 patient had an oral androgen receptor inhibitor, and 1 patient continued a 5-alpha-reductase during and after AHPT.
An additional 344 patients were enrolled on a second IRB-approved prospective trial of LR and IR patients from March 2011 to November 2015. This trial was less restrictive in its exclusion criteria. It included patients on alpha-blockers, and those with clinical stage T2c disease, diabetes mellitus, prostate volumes ≤100 cm3 and International Prostate Symptoms Score (IPSS) ≤25. The use of ADT was discouraged during treatment. Although 6 months of ADT after proton therapy was recommended in unfavorable intermediate-risk (UIR) patients and up to 2 years of ADT was allowed, all except 13 of these patients declined ADT over personal concerns about side effects and risks. There was no compelling evidence at the time of this trial on the timing and effectiveness of ADT in LR and IR prostate cancer. Therefore, to facilitate the evaluation of acute effects, ADT was discouraged. The main objective of these two trials was to determine whether moderately hypofractionated proton therapy for prostate cancer was safe and effective.
Finally, 23 patients are included in this analysis who were treated on an outcomes-tracking study with moderately AHPT outside of the two prior protocols, due to failure to meet entry criteria or deviation from protocol normal tissue constraints.
LR and IR patients typically had a maximum prescribed dose of 70 and 72.5 GyRBE at 2.5 GyRBE per fraction to the planning target volume (PTV), respectively, but could have a dose reduction to a minimum of 67.5 GyRBE if needed to meet OAR constraints. The recommended dose to the proximal seminal vesicles (SV) was 60 GyRBE, but a dose reduction to a minimum of 45 GyRBE was allowed to meet OAR constraints. Required staging included medical history, physical examination, complete blood count, testosterone level, PSA, alkaline phosphatase, computerized tomography and magnetic resonance imaging (MRI) (unless contraindicated) of the pelvis, chest X-ray, and ≥10-core biopsy (12-core preferred) within 6 months prior to study enrollment. The histologic diagnosis was confirmed by a second pathologist from our institution or a reference lab.
PSA values were obtained before and at the end of treatment, at 3-month intervals for 3 years, at 6-month intervals for an additional 2 years, and then annually. PSA progression was defined by the Phoenix criterion (nadir +2 ng/ml). Patients with PSA progression had a digital rectal exam, bone scan, and MRI of the pelvis and/or positron emission tomography (PET)–computed tomography (CT) to determine patterns of failure. Post-treatment prostate biopsy was performed only if local recurrence was suspected. Physician-verified toxicities were prospectively scored using the Common Terminology Criteria for Adverse Events, version 3.0 (CTCAE v3.0), weekly during treatment and then at 6-month intervals during the initial portion of this series, and with CTCAE v3.0 and v4.0 during the latter portion of the series. All adverse events were also classified retrospectively to include v4.0 to facilitate comparison with other series. Medical intervention as defined in the toxicity grading as inclusive of any prescription medication. IPSS was assessed before and at 6-month intervals after proton therapy.
Patient characteristics are given in . Eighty of the 269 LR patients (29.7%) were ‘very low risk’ according to the National Comprehensive Cancer Network Clinical Practice Guidelines. One hundred sixty-four of the 313 IR patients (52.4%) were considered ‘unfavorable’ on the basis of Gleason score 3 + 4 = 7 disease and ≥50% of cores positive for cancer, or the presence of multiple IR factors (cT2b-c, PSA 11–20, Gleason score 7), or a dominant Gleason pattern of 4.
Table 1. Patient and treatment characteristics.
Treatment simulation, planning, and delivery
The specifics of treatment simulation, planning, and delivery have been previously described [Citation10,Citation12]. All patients were treated with a rectal balloon or rectal saline to spare the rectum. We evaluated prostate motion utilizing Cine-MRI on a series of patients before the start of our prostate treatment program. PTV margins and compensator smearing were designed to account for range, density, and motion uncertainties. The plan for each field was evaluated to ensure 98% isodose coverage with a 3-mm proximal and a 5-mm distal margin on the PTV, and an intra-fraction motion was monitored for each patient. The planning target volume (PTV) expansion to account for potential set-up errors was 6 mm in the cranial-caudal axis and 4 mm axially. The clinical target volume (CTV) for LR patients was the prostate and, for IR patients, the prostate and proximal 1.5–2 cm of SVs. Patients were treated with passively scattered protons using opposed lateral or lateral-oblique fields, with one field per day. Treatment was with two fields per day only in patients where PTV coverage was not adequate with any single field, or where normal tissue constraints were not met with a single field per day plan. Patients were treated in a supine position, secured by a custom vacuum-locked bag. Image-guided treatment delivery was done with orthogonal kilovoltage imaging with alignment on intra-prostatic fiducial markers.
Normal-tissue dosimetric specifications
The rectal wall was defined from the inferior aspect of the ischial tuberosities to the sigmoid flexure using a 3-mm internal expansion from the outer surface of the rectal wall. The dose-constraint goals for the rectal wall were V45 < 50% and V65 < 30%. Minor and major deviations were defined as V45 of 50% to <60% and ≥60%, and/or V65 of 30% to <40% and ≥40%. A secondary rectal dose-constraint goal limited the absolute rectal volume receiving ≥70 GyRBE (Rec V70) to ≤10 cm3. This absolute rectal volume was defined as including both the rectal wall and rectal lumen.
The bladder wall was defined using a 3-mm internal expansion from the outer surface of the bladder wall. Dose-constraint goals for the bladder wall were V27.5 of <35 cm3, V70 < 13cm3, and V72.5 of <8 cm3. Minor and major deviations were classified as V27.5 of 35 to <45 cm3 and ≥45 cm3, and/or V70 of 13 cm3 to <15 cm3 and ≥15 cm3, and/or V72.5 of 8 to <10 cm3 and ≥10 cm3.
Treatment
A total of 269 LR patients were treated with 2.5 GyRBE per fraction to a median of 70.0 GyRBE (range, 64.4–72.5 GyRBE), including 1 VLR patient treated with 64.4 GyRBE, 4 LR patients treated with 67.5 GyRBE, and 1 LR patient treated with 72.5 GyRBE. A total of 313 IR patients were treated with 2.5 GyRBE per fraction to a median of 72.5 GyRBE (range, 67.5–72.5 GyRBE). Two of these patients received 67.5 GyRBE and 70 patients received 70 GyRBE to comply with the organs at risk (OAR) dose constraint goals. The SVs in IR patients were excluded from the target volume after 60 GyRBE, 57.5 GyRBE, 50.8 GyRBE, and 45 GyRBE in 90, 2, 1, and 2 patients, respectively, to comply with the OAR dose-constraint goals.
Statistical analysis
All statistical computations were performed with SAS and JMP software (SAS Institute, Cary, NC). The Kaplan-Meier product-limit method provided estimates of disease progression, survival, and freedom from toxicity. A log-rank test statistic assessed the level of statistical significance between strata of selected prognostic factors for these endpoints. All p-values below 0.05 were considered statistically significant. For the purposes of this analysis, the term FFBP includes all cases of disease progression, including a single patient with clinically detected biopsy-proven local recurrence without a rise in PSA that met the definition of biochemical recurrence.
Results
Survival and disease control
The overall median follow-up (combining toxicity and PSA data) was 8.0 years (range, 0.9–12.2). The median follow-up times were 8.1 years (range, 1.1–12.2) for LR patients and 7.5 years (range, 1.0–12.1) for IR patients. Overall, 554 patients (95.2%) were examined or contacted within 12 months of this analysis or were deceased. Furthermore, 505 (86.8%) had more than 5 years of available PSA data or failed prior to 5 years, and 329 (56.5%) had 7 years of available PSA data or failed prior to 7 years. A total of 558 (95.9%) had more than 5 years of clinical follow-up or died prior to 5 years, and 421 (72.3%) had 7 years of clinical follow-up or died prior to 7 years.
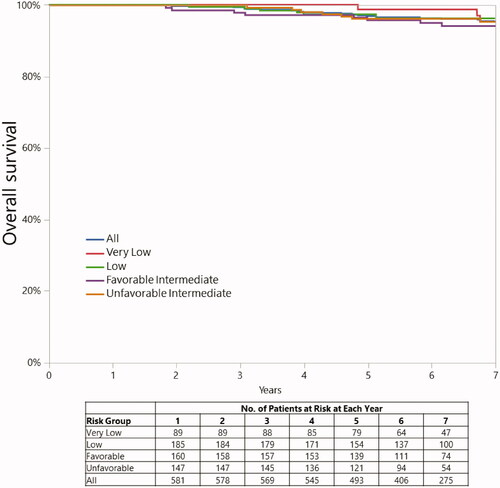
The 5-year overall survival rates for LR and IR patients were 97.7% (CI, 95.0%–99.0%), and 95.9% (CI, 92.5%–97.8%) while 7-year rates were 96.1% (CI, 93.2%–97.8%) and 94.8% (CI, 91.6%–96.9%), respectively (p = 0.6202) (). At 5 years, 6 LR and 10 IR patients had died of intercurrent disease; in addition, 2 intermediate-risk patients died of prostate cancer. At 7 years, 10 LR and 13 IR patients had died of intercurrent disease; there were no additional deaths from prostate cancer between 5 and 7 years.
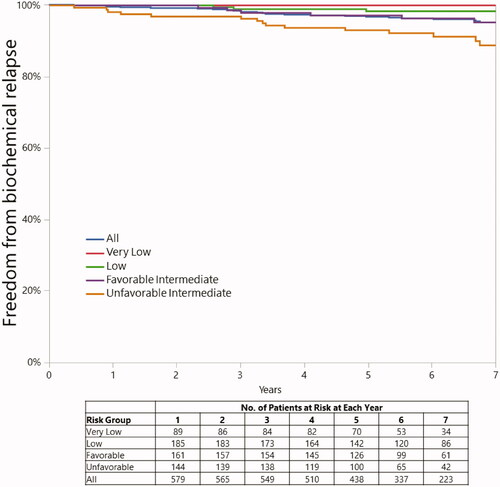
Eighteen patients (3 LR and 15 IR) had disease progression at 5 years. Five-year rates of FFBP were 96.8% (CI, 94.9%–98.0%), 98.8% (CI, 96.4%–99.6%), and 95.0% (CI, 91.9%–97.0%) in the overall group and LR and IR subsets, respectively. The 5-year rates of FFBP were 100% (CI, not available) and 98.3% (CI, 94.8%–99.4%) for the VLR and LR subsets. The 5-year rates of FFBP were 97.2% (CI, 92.7%–98.9%) and 93.1% (CI, 87.9%–96.1%) for the FIR and UIR subsets ().
Twenty-four patients (3 LR and 21 IR) had disease progression at 7 years. Seven-year rates of FFBP were 95.2% (CI, 92.9%–96.8%), 98.8% (CI, 96.4%–99.6%), and 91.9% (CI, 87.8%–94.7%) in the overall group and the LR and IR subsets, respectively. The 7-year rates of FFBP were 100% (CI, not available) and 98.3% (CI, 94.8%–99.4) for the VLR and LR subsets. The 7-year rates of FFBP were 95.2% (CI, 89.5%–97.9%) and 88.8% (CI, 81.9%–93.3%) for the FIR and UIR subsets ().
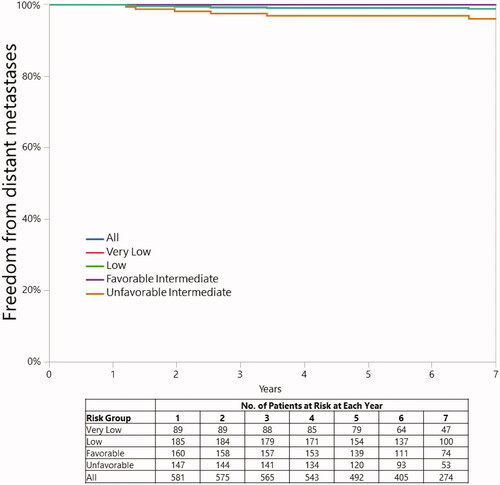
The 5 and 7-year rates of freedom from distant metastases were 100.0% and 100.0% in the LR and 98.4% and 97.9% in the IR patients (p = 0.0050), (respectively). They were 100.0 and 100.0% in FIR patients and 96.9 and 96.0% in UIR patients, respectively (). On univariate analysis, there was no association between a dose of 70 GyRBE versus 72.5 GyRBE and disease recurrence in the IR group as a whole (p = 0.1524) or in the UIR group (p = 0.3521).
Patterns of PSA response and disease progression
PSA progression occurred in 26 patients, and 1 additional patient with an isolated clinically detected biopsy-proven local failure and a PSA rise that did not meet the definition of PSA failure. Three failures occurred after 7 years. Of the 26 patients with PSA progression, 2 had PSA progression alone, 6 also had local recurrence alone (4 with positive prostate biopsy and 2 with MRI or PET-fluciclovine-F18 findings consistent with local recurrence), 10 had a regional failure, 3 had a regional and distant failure, and 5 had a distant failure. There were no failures in patients with very LR disease. Disease progression occurred in 4 (2.1%) of 189 LR patients, 6 (4.0%) of 149 favorable IR patients, and 17 (10.4%) of 164 unfavorable IR patients.
Toxicity
The incidences of acute (within 6 months) radiation-related CTCAE v4.0 grade 2 and grade 3 or higher gastrointestinal (GI) toxicity were <0.1% and <0.1%, respectively. Actuarial 5- and 7-year rates of late (after 6 months) radiation-related CTCAE v4.0 grade 2+ GI toxicities were 9.9 and 11.2%, respectively, including 43 patients with grade 2+ rectal bleeding (3 patients with grade 3), 13 patients with grade 2+ proctitis (3 patients with grade 3 toxicity, 2 of whom developed anal fistulae), and 7 patients with both (no patients with grade 3 toxicity). Five patients had other GI grade 2+ problems including anal incontinence and anal fistula (2 patients with grade 3 toxicity, one of which was the anal fistula). Two grade 2 bleeding events occurred after 7 years. On univariate analysis, the CTCAE v4.0 late grade 2 GI toxicity correlated significantly with the Rec V70 (with a cutpoint of 9.0 cm3) (p = 0.0002). There was no significant correlation with dose, age (greater or less than 60), prostate volume on ultrasound (greater or less than the median volume of 36 cm3), diabetes, heart disease, smoking (greater or less than a 20 pack/year history), aspirin, or LR vs IR disease.
The corresponding 5- and 7-year rates of late grade 3 GI toxicities were 0.8 and 0.8% for LR, 2.0 and 2.0% for IR patients, and 1.4 and 1.4% overall, respectively. There was no significant difference in late CTCAE v4.0 grade 3 toxicity for patients treated from April 2008 through October 2011 with more restrictive exclusion criteria versus those treated after October 2011. Late CTCAE v4.0 grade 3 GI toxicity was significantly related to Rec V70 using an optimized cutpoint of 6.8 cm3 (p = 0.0142). The 5-year risk of grade 3 GI toxicity for Rec V70 < 6.8 cm3 was 0.5% (95% CI: 0.1%–2.2%) and 3.1% (95% CI: 1.4%–6.6%) for Rec V70 ≥ 6.8 cm3. Three patients developed persistent rectourethral fistulae. One patient was treated to 72.5 GyRBE with a Rec v70 of 17.3 cm3. He had transfusions and a rectal suture procedure for rectal ulceration with bleeding before progressing to abscess and fistula formation with the bladder. This was managed with a diverting colostomy and a foley catheter. The second patient was treated with 72.5 GyRBE with a Rec v70 of 6.9 cm3. He had painful rectal ulceration managed with hyperbaric oxygen treatment and subsequent diverting colostomy before developing the rectourethral fistula. Attempted closure with a gracilis flap was unsuccessful, and there was no further surgical intervention. The third patient was treated with 70 GyRBE with a Rec v70 of 6.8 cm3. He developed a rectourethral fistula with pain and recurrent urinary tract infections managed initially with a colostomy and suprapubic catheter placement, but eventually with a total pelvic exenteration with a sigmoid urinary conduit. All other late grade 3 GI toxicities were transient.
The incidence of acute (within 6 months) and late CTCAE v4.0 grade 3 or higher GU toxicity was 0.2%. The 5- and 7-year rates of late radiation-related CTCAE v4.0 grade 3 GU toxicities were 1.3% and 2.1%, respectively. The 3 patients with late rectourethral fistulae were coded as having both grade 3 GI and GU complications and are the only patients with persistent Gr 3 GU toxicities. There were no CTCAE v4.0 grade 4 or higher late GU toxicities. On univariate analysis, there was no significant correlation of grade 3 or higher GU events with Rec V70, IR versus LR disease, dose, age (greater or less than 60), prostate volume on ultrasound (greater or less than the median volume of 36 cm3), diabetes, heart disease, smoking (greater or less than a 20 pack/year history), or aspirin.
Patient-reported outcomes
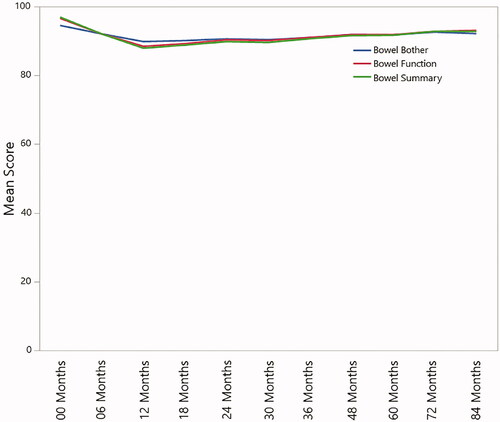
EPIC bowel function and bother scores at baseline, 5 years, and 7 years were available on 574, 300, and 191 patients, respectively. IPSS at baseline, 5 years, and 7 years were available on 579, 298, and 191 patients, respectively. The mean and standard deviation (SD) of the IPSS and EPIC bowel function and bother scores were 7 (SD = 5), 97 (SD = 7), and 94 (SD = 6), respectively at baseline, 7 (SD = 5), 92 (SD = 13), and 92 (SD = 9) at the 5-year follow-up, and 7 (SD = 5), 93 (SD = 12), and 92 (SD = 10) at the 7-year follow-up () At 7+ years follow-up, 2.1% (4/190) of patients reported a moderate or greater problem with “losing control of your stools,” compared to a pretreatment baseline rate of 0.4% (2/565). At 7+ years follow-up, 2.5% (4/190) of patients reported a moderate problem, and no patient reported a big problem, with bloody stools. By comparison, the pretreatment baseline rate was 0.2% (1/565) of patients reporting a big problem, but no patient reporting a moderate problem, with bloody stools.
Figure 4. EPIC scores and scales for specific questions regarding fecal incontinence and rectal bleeding over time.
There was no significant difference in 5- and 7-year IPSS and EPIC bowel function and bother scores for patients treated between April 2008 and October 2011 with more restrictive exclusion criteria, versus those treated after October 2011.
Discussion
The outcomes in this study compare favorably with those seen in our experience with conventionally fractionated proton therapy for prostate cancer, and with other published data with moderately accelerated hypofractionated proton and photon radiation.
Comparison of control rates
This study had a 5-year FFBP rate of 98.8% for LR and 95.0% for IR disease, with 97.2% in FIR and 93.1% in UIR disease. Only 7% of the UIR patients were treated with ADT. By comparison, the 5-year FFBP in our initial two prospective protocols for conventionally fractionated proton therapy for prostate cancer was 99% for 89 patients with LR and 99% for 82 patients with IR disease [Citation13]. Prior ADT was allowed in these protocols, and 11% of patients had undergone ADT, but concurrent or adjuvant ADT was not allowed. The results in the current series closely resemble a larger review of the first 1327 patients treated with conventionally fractionated proton therapy for prostate cancer at our institution and included 546 patients with LR and 554 patients with IR disease [Citation14]. The 5-year FFBP rates were 99% for LR and 94% for IR disease, with only 10% of IR patients treated with ADT.
In comparing other series utilizing the same moderately hypofractionation schedule of 70 GyRBE at 2.5 GyRBE per fraction, results are very similar. The control rates in this report are also similar to those from the University of Pennsylvania series of 184 LR and IR prostate cancer patients treated with moderately hypofractionated proton therapy (70 GyRBE at 2.5 GyRBE per fraction) [Citation7]. The 4-year freedom from clinical and biochemical progression was 94.4% in LR, 92.5% in FIR, and 93.8% in UIR disease. Our 5-year FFBP of 93.1% in UIR patients compares very favorably, considering only 7% (12/164) of these patients had ADT, compared with 48% of the UIR patients in the University of Pennsylvania series. This difference may be related to our treatment of IR patients to 72.5 GyRBE as compared to the 70 GyRBE used at the University of Pennsylvania.
However, in comparison with photon therapy, the NRG Oncology/Radiation Therapy Oncology Group (RTOG) randomized trial of moderately hypofractionated versus conventionally fractionated photon radiation for prostate cancer included 1,115 patients with LR prostate cancer followed for a median of 5.8 years [Citation2]. The hypofractionated arm of this trial utilized 70 Gy in 28 fractions and had a 5-year disease-free survival rate of 86.3%. The Cleveland Clinic series using this same IMRT hypofraction schedule reported 5-year biochemical relapse-free survival rates of 96%, 86%, and 82% for LR, FIR and UIR patients [Citation15]. Comparison with the FFBP rates in our series raises the question of whether protons may be more effective than photons, due to the predominant double-stranded versus single-stranded DNA damage, or a possible underestimate of the historical relative RBE factor of 1.1 [Citation16,Citation17]. Improvements in imaging for staging in our series may have also influenced the difference in FFBP.
Other series have explored slightly different moderate hypofractionation schedules including the Loma Linda proton trial and the CHHiP photon trial [Citation3,Citation18]. The Loma Linda study included 142 LR and 4 IR prostate cancer patients treated with 60 GyRBE at 3 GyRBE/fraction with a median follow-up of 42 months. The 3- and 5-year biochemical progression-free survival rates were 99.3% and 97.9% respectively. The CHHiP randomized multicenter IMRT trial included 1074 men treated on a 60-Gy at 3 Gy per fraction arm whose 5-year biochemical and clinical failure-free (BCFF) survival rate of 90.6% was non-inferior to the 88.3% rate for 1,065 patients treated on a 74-Gy at 2 Gy per fraction arm. The third arm including 1077 patients treated with 57 Gy at 3 Gy per fraction had a BCFF rate that was inferior compared with the 60-Gy arm, and was not non-inferior to that of the 74-Gy arm. The 5-year BCFF rate for LR and IR patients were 96.6% and 90.2% for those treated on the 60-Gy arm, and 96.7% and 92.3% for those treated on 74-Gy arm. The median follow-up was 62.4 months for all patients included in this trial.
Comparison of toxicities
The GI and GU toxicity rates were low in this series with 5-year CTCAE v4.0 late grade 3 GU toxicity of 1.3%, and the late grade 2 and 3 GI toxicities of 9.9% and 1.4%, respectively. There were no grade 4 or higher toxicities. We found a significant correlation between the Rec V70 and late grade 3 GI toxicity, with only one grade 3 event occurring below a Rec V70 of 6.8 cm3. These outcomes are similar to those reported from our institution () with conventionally fractionated proton therapy in our initial three prospective protocols for LR, IR, and HR disease, and in our retrospective review of the first 1327 patients [Citation13,Citation14].
Table 2. Literature review.
The University of Pennsylvania series reports similar toxicity outcomes with a cumulative 4-year incidence of CTCAE v4.0 late grade 2 GI toxicity rate of 13.6%, with 1 late grade 3 GI toxicity. There were no late grade 3 or 4 GU events reported [Citation7].
The NRG/RTOG trial reported toxicity according to CTCAE v3.0. The moderate hypofractionation arm had a late grade 3 GU toxicity rate of 3.5% and late grade 2 and 3 GI toxicity rates of 18.3% and 4.1% [Citation2]. These are higher toxicity rates than seen in this study of proton therapy or in the University of Pennsylvania series. However, 20% of the patients in the NRG/RTOG study were treated with 3-dimensional conformal x-rays and 80% with IMRT. The Cleveland Clinic trial reported very low rates of IMRT toxicity scored with CTCAE v4.03. A recent pooled toxicity analysis compared moderately hypofractionated IMRT and proton therapy [Citation11]. This study showed a non-significant trend toward higher grade 2 GI toxicity with proton therapy (14.6%) versus IMRT (4.7%), but lower grade 3 GI and GU toxicity with proton therapy (1.6% and 0.4%) versus IMRT (3.7% and 1.1%). In contrast to the current prospective study with relatively complete follow-up, this pooled analysis was a retrospective review with a relatively long accrual time of 20 years compared with the median follow-up of 6.67 years for IMRT patients and 3.66 years for proton therapy patients.
The Loma Linda moderately hypofractionated proton trial utilized 60 GyRBE at 3 GyRBE/fraction with two parallel opposed proton beams with both fields treated per day. The 3-year actuarial rate of CTCAE version 4.0 grade 3 GU toxicity was 0.7%, and the grade 2 and 3 GI toxicities were 5.1% and 0%, respectively. Comparison of toxicities with the CHHiP trial is limited by the fact that the CTCAE scoring was not used in this study. However, with the RTOG scoring scale, the 5-year incidence of grade 3 GU and GI toxicities were <1% versus <1%, and <1% versus 0%, for the 60 Gy and 74 Gy arms, respectively. The 5-year estimated cumulative incidence of grade 3 or greater GU and GI toxicities were 6% versus 3%, and 3% versus 2%, for the 60-Gy and 74-Gy arms respectively.
Patient reported outcomes
The mean IPSS of 7 at baseline remained unchanged at the 5- and 7-year follow-up time points. The mean EPIC bowel function score of 97 at baseline decreased modestly to a score of 92 at 5 years and 93 at 7 years. The mean EPIC bowel bothers score remained relatively stable with values of 94 at baseline and 92 at both 5 and 7 years.
The 5-year PRO values in our series compare favorably with those seen in conventionally fractionated proton series. Bryant et al. reported a median IPSS at 5-years follow-up that remained stable at 7 years in the conventionally fractionated proton series from our institution. The mean EPIC summary scores for bowel, urinary irritative/obstructive, and urinary incontinence domains remained relatively stable as well.
The University of Pennsylvania conventionally fractionated proton series reported a median IPSS of 8.1 at baseline and 8.9 at 4 years follow-up [Citation7]. They saw a decline in EPIC scores at 1 year follow-up, but no significant differences at 4 years follow-up as compared with baseline.
There are two ongoing national trials comparing IMRT and proton therapy—the COMPPARE and PARTIQoL trials—each with different primary endpoints. IMRT inherently irradiates the entire circumference of the rectum with moderate doses of radiation, and some measure of post-radiation fibrosis might impact rectal distensibility and function. In contrast, proton therapy has the ability to avoid radiation altogether to a large portion of the rectal circumference. Hoppe et al. compared PRO for proton therapy versus IMRT and found that IMRT patients were more likely to report “moderate” or “big problems” with rectal urgency (p = 0.02) or bowel frequency (p = 0.5) [Citation6]. This study informed the design and primary endpoint of the ongoing COMPPARE trial, which is a comparison of bowel urgency and frequency between proton therapy, including moderately AHPT, and IMRT. In contrast, the primary endpoint for the PARTIQoL study is the bowel summary score, which is heavily dominated by rectal bleeding, a complication related to dose only to the anterior rectal wall. It is unlikely that this study will demonstrate a difference between proton therapy and IMRT since the dose to the anterior rectal wall is not different between these two treatment modalities. In addition, the relevance of this endpoint could be questioned since the complication of rectal bleeding is almost completely eliminated with rectal spacers [Citation19].
Study limitations and strengths
All patients in this study were treated with passively scattered proton therapy. The newer approach with pencil-beam proton therapy provides a more conformal treatment that may lead to a reduction in toxicity. This study is limited by potential selection bias related to patient choice for proton therapy and access to care, the selected exclusion criteria, and the lack of comparison arms with photon irradiation. Local control rates may be overestimated because post-treatment prostate biopsy was done only when isolated disease recurrence was suspected.
Strengths of this study include a large number of patients treated over a relatively short period of time, a consistent treatment policy with techniques based on institutional policy, consistent follow-up, and prospective assessment by experienced staff.
Conclusions
Five-year outcomes with image-guided moderately AHPT for LR- and IR prostate cancer include high efficacy and minimal toxicity, and compare favorably to published outcomes achieved with hypofractionated photon therapy as well as both standard and hypofractionated proton therapy. Image-guided moderately AHPT appears to be a viable treatment approach for men with LR or IR prostate cancer.
Supplemental Material
Download MS Word (29.3 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors agree to share the data upon reasonable request by researchers.
References
- Morgan SC, Hoffman K, Loblaw DA, et al. Hypofractionated radiation therapy for localized prostate cancer: an ASTRO, ASCO, and AUA evidence-based guideline. J Urol. 2019;201(3):528–534.
- Lee WR, Dignam JJ, Amin MB, et al. Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol. 2016;34(20):2325–2332.
- Dearnaley D, Syndikus I, Mossop H, CHHiP Investigators, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17(8):1047–1060.
- Kupelian PA, Willoughby TR, Reddy CA, et al. Hypofractionated intensity-modulated radiotherapy (70 Gy at 2.5 Gy per fraction) for localized prostate cancer: Cleveland clinic experience. Int J Radiat Oncol Biol Phys. 2007;68(5):1424–1430.
- Catton CN, Lukka H, Gu CS, et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol. 2017;35(17):1884–1890.
- Hoppe BS, Michalski JM, Mendenhall NP, et al. Comparative effectiveness study of patient-reported outcomes after proton therapy or intensity-modulated radiotherapy for prostate cancer. Cancer. 2014;120(7):1076–1082.
- Grewal AS, Schonewolf C, Min EJ, et al. Four-Year outcomes from a prospective phase II clinical trial of moderately hypofractionated proton therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2019;105(4):713–722.
- University of Florida. A Prospective Comparative Study of Outcomes With Proton and Photon Radiation in Prostate Cancer (COMPPARE) clinicaltrials.gov2018. [cited January 8, 2021]. Available from: https://clinicaltrials.gov/ct2/show/NCT03561220.
- Massachusetts General Hospital. Proton Therapy vs. IMRT for Low or Intermediate Risk Prostate Cancer (PARTIQoL) clinicaltrials.gov2012. [cited January 8, 2021]. Available from: https://clinicaltrials.gov/ct2/show/NCT01617161.
- Henderson RH, Bryant C, Hoppe BS, et al. Five-year outcomes from a prospective trial of image-guided accelerated hypofractionated proton therapy for prostate cancer. Acta Oncol. 2017;56(7):963–970.
- Vapiwala N, Wong JK, Handorf E, et al. A pooled toxicity analysis of moderately hypofractionated proton beam therapy and intensity-modulated radiation therapy in early stage prostate cancer patients. Int J Radiat Oncol Biol Phys. 2021;110(4):1082–1089.
- Mendenhall NP, Li Z, Hoppe BS, et al. Early outcomes from three prospective trials of image-guided proton therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2012;82(1):213–221.
- Mendenhall NP, Hoppe BS, Nichols RC, et al. Five-year outcomes from 3 prospective trials of image-guided proton therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2014;88(3):596–602.
- Bryant C, Smith TL, Henderson RH, et al. Five-Year biochemical results, toxicity, and Patient-Reported quality of life after delivery of dose-escalated image guided proton therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2016;95(1):422–434.
- Abu-Gheida I, Reddy CA, Kotecha R, et al. Ten-Year outcomes of moderately hypofractionated (70 Gy in 28 fractions) intensity modulated radiation therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2019;104(2):325–333.
- Matsumoto Y, Ando K, Kato TA, et al. Difference in degree of sub-lethal damage recovery between clinical proton beams and X-rays. Radiat Prot Dosimetry. 2019;183(1–2):93–97.
- Paganetti H. Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Phys Med Biol. 2014;59(22):R419–72.
- Slater JM, Slater JD, Kang JI, et al. Hypofractionated proton therapy in early prostate cancer: results of a phase I/II trial at Loma Linda University. Int J Part Ther. 2019;6(1):1–9. DOI:https://doi.org/10.14338/IJPT-19-00057
- Hamstra DA, Mariados N, Sylvester J, et al. Continued benefit to rectal separation for prostate radiation therapy: final results of a phase III trial. Int J Radiat Oncol Biol Phys. 2017;97(5):976–985.