Abstract
Background
The diagnosis of pancreatic ductal adenocarcinoma (PDAC) has an enormous impact on patients, and even more so if they are of younger age. It is unclear how their treatment and outcome compare to older patients. This study compares clinicopathological characteristics and overall survival (OS) of PDAC patients aged <60 years to older PDAC patients.
Method
This is a retrospective, population-based cohort study using Netherlands Cancer Registry data of patients diagnosed with PDAC (1 January 2015–31 December 2018). Kaplan–Meier curves and Cox proportional hazards models were used to assess OS.
Results
Overall, 10,298 patients were included, of whom 1551 (15%) were <60 years. Patients <60 years were more often male, had better performance status, less comorbidities and less stage I disease, and more often received anticancer treatment (67 vs. 33%, p < 0.001) than older patients. Patients <60 years underwent resection of the tumour more often (22 vs. 14%p < 0.001), more often received chemotherapy, and had a better median OS (6.9 vs. 3.3 months, p < 0.001) compared to older patients. No differences in median OS were demonstrated between both age groups of patients who underwent resection (19.7 vs. 19.4 months, p = 0.123), received chemotherapy alone (7.8 vs. 8.5 months, p = 0.191), or received no anticancer treatment (1.8 vs. 1.9 months, p = 0.600). Patients <60 years with stage-IV disease receiving chemotherapy had a somewhat better OS (7.5 vs. 6.3 months, p = 0.026).
Conclusion
Patients with PDAC <60 years more often underwent resection despite less stage I disease and had superior OS. Stratified for treatment, however, survival was largely similar.
Introduction
Survival after pancreatic ductal adenocarcinoma (PDAC) remains poor, with a median overall survival (OS) of 3.5 months among all patients [Citation1]. Unfortunately, little improvement in survival has been achieved over time [Citation1,Citation2]. This is largely attributable to the aggressive tumour biology and considerable resistance to systemic treatments of PDAC [Citation3]. Interestingly, only in men <65 years and women <50 years with PDAC a decline of overall cancer mortality has been observed over the last years in the European Union [Citation4]. Approximately 20% of the PDAC patients are younger than 60 years of age at the time of diagnosis [Citation1,Citation5]. Despite being a minority of patients, potential years-of-life-lost by PDAC in young patients account for a substantial proportion of the total disease burden [Citation6]. Previous studies in the Netherlands explored trends in pancreatic cancer care and survival [Citation1,Citation7] or focussed on other sub-groups (e.g., locally advanced pancreatic cancer [Citation8] or octogenarians [Citation9]). Clinical characteristics and survival of young patients with PDAC have not been explored.
The association between younger age and survival of PDAC remains debated, as population-based studies report varying results [Citation10–13]. Differences in molecular tumour biology could affect survival outcomes in younger and older patients with PDAC. For example, a higher rate of KRAS wild-type tumours has been reported in younger PDAC patients [Citation14–16]. In addition, differences in disease presentation and treatment have been described between age groups and might affect survival outcomes. For example, pancreatoduodenectomy is performed less frequently in older patients [Citation1], while this is considered safe [Citation17,Citation18]. Whether resection or chemotherapy offers a greater survival benefit in younger patients than in older patients is unclear, as this has not been studied in previous studies. Therefore, we aimed to compare clinical characteristics, treatment, and survival of patients with PDAC <60 years of age to older patients.
Methods
Study design
This was a retrospective observational cohort study using population-based data obtained from the Netherlands Cancer Registry (NCR). Newly diagnosed cancer patients in the Netherlands were identified through linkage to the national pathological archive (PALGA) and supplemented with notifications from the National Registry of Hospital Care in case of no pathological verification. Details on demographics, clinicopathological characteristics, and treatment were obtained from electronic patient files by trained personnel of the NCR. The vital status of included patients was determined through annual linkage with the Municipal Personal Records Database. The study was approved by the NCR review board and the scientific committee of the Dutch Pancreatic Cancer Group [Citation19]. Retrospective population-based data in the NCR were provided anonymised, approval of a medical ethics committee was not required under Dutch law. This study was reported following the STROBE Statement (Supplemental File 1) [Citation20].
Study population
Adult patients diagnosed with PDAC between January 2015 until December 2018 in the Netherlands were included in this study. PDAC was defined by International Classification of Disease-Oncology (ICD-O-3: C25 excluding C25.4; morphology codes 8000, 8010, 8012, 8020, 8140, 8141, 8260, 8310, 8440, 8480, 8481, 8490, 8500, 8560). Exclusion criteria were diagnoses at autopsy, diagnosis or cancer treatment abroad, or younger than 18 years at diagnosis.
Definitions
Patients were divided into age groups; <60 or ≥60 years at the time of diagnosis because these groups have not been previously explored. In addition, a group of early-onset PDAC was defined as <50 years of age. Tumour stage was based on the pathological tumour-node-metastasis (TNM) classification according to the valid Union for International Cancer Control (UICC) edition (2015–2016: 7th, 2017–2018: 8th), or based on the clinical TNM in case of neoadjuvant treatment or if no pTNM was available (no resection). Location of the primary tumour was classified as the pancreatic head (C25.0), body (C25.1), tail (C25.2), or other (C25.3–C25.9). The performance status of patients was described according to the WHO scale from zero to four and grouped 0–1 (good), 2 and 3–4 (poor). The reason for no anticancer treatment was derived from reports of the treating clinician and registered in the NCR. OS was defined as the time interval between the date of diagnosis and the date of death (all causes) or censored if they were alive at the last date of follow-up (1 February 2020). The date of diagnosis is based on the first date of pathological confirmation of cancer. If no pathological confirmation was available, the date of first hospital admission was used in which this cancer was diagnosed. If no hospital admission was available, the date of diagnosis on imaging was used.
For each patient, the hospital of the first diagnosis (i.e., hospital of clinical or suspected diagnosis) was determined, as well as the main hospital of treatment (ordered by the hospital of resection, systemic treatment, other cancer treatment, diagnosis in case of non-cancer treatment). Hospitals were divided into academic vs. non-academic.
Statistical analysis
Continuous variables were expressed as a mean ± standard deviation or as a median ± interquartile range, depending on their distribution (normal or not normal). For univariable analysis, continuous variables were compared using a t-test or a Mann–Whitney U test. Categorical variables were assessed using the Chi-squared test or Fisher’s exact test.
OS was calculated using Kaplan–Meier estimates, and log-rank tests were used to compare survival curves. For regression models assumptions on the linearity of continuous variables were checked. A Cox proportional hazard regression model was used to calculate hazard ratios and determine independent predictors of OS. Variables entered in the multivariable model were age, sex, and based on univariable Cox regression models (p < 0.10). A multivariable logistic regression model was constructed to determine whether age was an independent predictor of receiving anticancer treatment. Age was entered as a categorical variable due to non-linearity.
Two-sided p-values <0.05 were considered statistically significant. Missing data were handled using multiple imputations using the MICE package creating five imputed datasets. Regression analyses were performed on these five imputed datasets, after which results were pooled. Overall, multiple imputations did not alter the conclusions of the regression models. R statistical software (version 4.1.1.; www.r-project.org) was used for all statistical analyses.
Results
Patient characteristics
A total of 10,298 patients were diagnosed with PDAC, of whom 1551 patients (15%) were <60 years. Patient characteristics are described in . The median age of the total cohort was 72 years (IQR 64–79). Younger patients were more often male, had a better performance status, lower Charlson Comorbidity Index, and were diagnosed less often with stage I disease. There were no other differences suggestive of different tumour biology, such as in tumour size, differentiation, or location. Patients aged <60 years were more likely to receive anticancer treatment compared to older patients (67 vs. 33%, p < 0.001), also in multivariable analysis younger patients were more likely to receive anticancer treatment than older patients (Supplementary Table 1). Patients <60 years underwent resection of the tumour in 22% of the patients, compared to 14% in the older patients (p < 0.001), and more often received (neo)adjuvant and palliative chemotherapy, particularly FOLFIRINOX ().
Table 1. Patient and treatment characteristics of patients diagnosed with pancreatic ductal adenocarcinoma in the Netherlands in 2015 until 2018 (n = 10,298).
Survival analysis
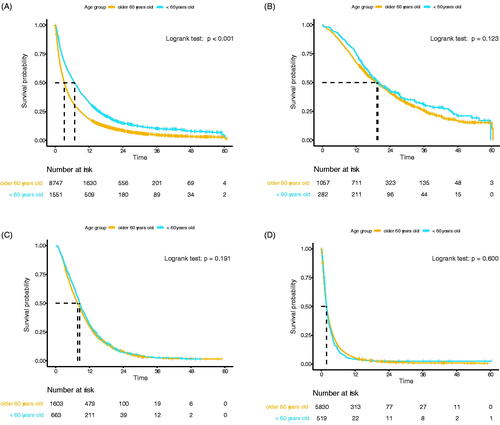
Patients <60 years had a significantly better OS compared to their older counterparts (median OS 6.9 vs. 3.3 months, p < 0.001, ). Older age was an independent prognostic factor of worse OS in multivariable analysis (HR 1.01, 95% CI 1.00–1.01, p < 0.001, Supplementary Table 2). No difference in median OS was observed between age groups for patients who underwent immediate resection (<60 vs. ≥60 years: 19.7 vs. 19.4 months, p = 0.123, ) and who received systemic treatment only (7.8 vs. 8.5 months, p = 0.191, ). In stage IV patients who received chemotherapy, patients <60 years had a slightly better OS compared to older patients (7.5 vs. 6.3 months, p = 0.026), while this could not be demonstrated for stage I-III patients (11.4 vs. 11.4 months, p = 0.941). In patients who did not undergo any anticancer treatment no difference was demonstrated (median OS 1.8 vs. 1.9 months, p = 0.600, ). In both age groups, the most frequent reasons for not receiving any cancer treatment was due to the patient’s preference, condition of the patient, and extensive disease, respectively ().
Figure 1. (A) Overall survival of the total cohort (n = 10,298). (B) Overall survival in patients who underwent immediate resection of the primary tumour (n = 1339). (C) Overall survival of patients who received chemotherapy, without resection (n = 2266). (D) Overall survival of patients who received no anticancer treatment (n = 6349).
Academic hospital of diagnosis
A total of 869 patients (8.4%) were diagnosed in an academic hospital, 155 (11%) of 1551 patients <60 years, and 714 (8.2%) of 8747 patients of older age (p = 0.019). Among patients <60 years, diagnosis in an academic hospital was associated with more stage I disease (23 vs. 11%, p < 0.001), previous history of malignancy (19 vs. 8%, p < 0.001), a higher Charlson Comorbidity index, and a higher proportion that underwent resection (34 vs. 21%, p < 0.001) (Supplementary Table 3). Diagnosis in an academic hospital was associated with an improved median OS (10.6 vs. 6.5 months, p = 0.002, ). However, in multivariable Cox regression, this association was not confirmed when the disease stage was included (HR (academic vs. non-academic) = 0.95, 95%CI 0.77–1.17, ).
Figure 2. Overall survival of patients <60 years old diagnosed in an academic hospital vs. diagnosed in a non-academic hospital (n = 1551).
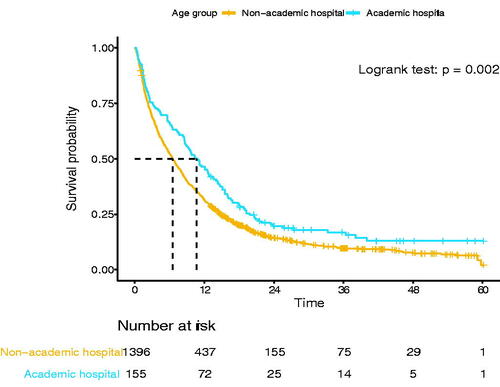
Table 2. Multivariable Cox proportional hazards model of patients diagnosed with pancreatic cancer at <60 years in the Netherlands from 2015 until 2018 (n = 1551).
Patients <50 years
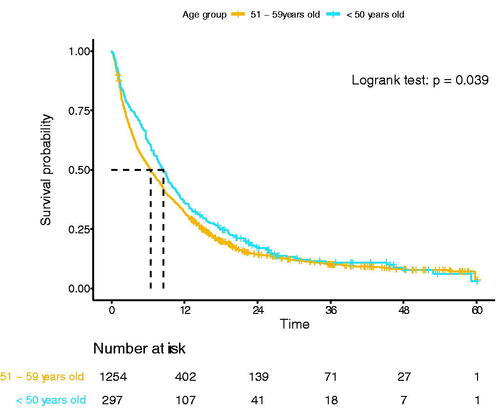
Overall, 297 patients (2.3%) were younger than 50 years at the time of diagnosis (n = 273, 40–49 years, n = 21, 30–39 years, and n = 3, 18–29 years). Compared to patients 50–59 years, patients <50 years more often received anticancer treatment (79 vs. 57%, p < 0001), other clinicopathological characteristics were comparable (). Younger patients had a better median OS compared to patients aged 50–59 years (8.5 vs. 6.5 months, p = 0.039, ). However, when stratified for treatment, no survival difference could be demonstrated for patients who underwent resection (22.2 vs. 19.0 months, p = 0.461), underwent chemotherapy only (8.4 vs. 8.5 months, p = 0.658), and received no anticancer treatment (1.4 vs. 1.9 months, p = 0.939) (Supplemental Figures 1(A–C)).
Table 3. Patient and treatment characteristics of patients with pancreatic ductal adenocarcinoma aged 51–59 years old compared to patients <50 years old at time of diagnosis in the Netherlands in 2015 until 2018 (n = 1551).
Discussion
This population-based cohort study found that patients with PDAC <60 years had less stage I disease and more often underwent surgical resection, with superior survival compared to older patients. Stratified for anticancer treatment, however, no difference in OS was found between age groups, except for treated patients with stage IV disease. It appears that the superior prognosis of age <60 years is largely attributable to differences in treatment rather than tumour stage or clinicopathological factors. Age should not be a key determinant of treatment.
Clinical characteristics and survival of young PDAC patients have not been investigated in a European population recently. Patients <60 years had less stage I disease at the time of diagnosis compared to older patients. Two previous studies also reported an association between younger age at diagnosis of PDAC (<40 or <50 years) and more advanced stage of disease [Citation10,Citation11]. This observation is possibly related to a delay in diagnosis as there might be a lower suspicion of PDAC and reluctance in seeking medical care. Moreover, the advanced stage at the time of diagnosis in young patients could also be related to tumour biology. In young patients diagnosed with PDAC (<50 years) more pathogenic variants in other oncogenic drivers than KRAS have been described [Citation16,Citation21]. Such molecular differences could represent different tumour biology. In other cancers, such as in colorectal cancer patients <50 years [Citation22,Citation23], more poorly differentiated, aggressive tumours are described compared to older patients. However, no difference in tumour size or differentiation grade could be demonstrated between age groups in our study. Family history of cancer may suggest different oncogenic drivers in young patients, such as BRCA1/2 [Citation24,Citation25]. Unfortunately, family history was not recorded in the NCR. However, a large previous study found no association between young age of diagnosis (<40 years) of PDAC and family history of malignancy in 36,145 patients in Japan [Citation11]. To investigate the molecular characteristics of patients ≤60 years with PDAC, a multicentre prospective cohort study in the Netherlands is currently recruiting patients who undergo extensive molecular characterisation of the tumour (PAN-NGS study, NL9040). Additionally, less advanced disease in older patients might be due to fewer diagnostic investigations if patients were not eligible for any anticancer treatment because of a poor performance status or more comorbid diseases.
Surgical resection remains the cornerstone of treatment for PDAC in patients with localised disease and is associated with a better OS compared to other treatment modalities. Patients <60 years more often undergo resection compared to older patients (22 vs. 14%). Two large studies previously demonstrated an improved OS in younger patients (<40 and <50 years) compared to older patients [Citation10,Citation11]. However, when stratified for resection, these studies show conflicting results. Eguchi et al. found no survival difference between patients <40 years and older patients, whereas Ordonez et al. found a survival advantage for PDAC <50 years old (27.3 vs. 24.3 months; p < 0.001). Prior studies did not stratify for other treatments than resection [Citation10–13]. In our study, no survival differences were seen in patients with the resected disease, or other treatment subgroups (chemotherapy only or no anticancer treatment). This persisted in patients <50 years old. Therefore, older patients should be considered for surgery if the tumour is deemed resectable.
Only in patients <60 years with stage IV disease who received chemotherapy, OS was better compared to older patients, which might be related to more intensive treatment regimens in young patients, such as FOLFIRINOX. In stage I–III disease, younger patients responding well to chemotherapy possibly underwent resection more often than older patients, which may have reduced a survival difference in patients who underwent resection or received chemotherapy only. Yet, no details about the response on chemotherapy or extent of vessel involvement were available.
In our study, 15% of the patients diagnosed with PDAC in the Netherlands were <60 years. Early-onset PDAC (<50 years) occurred in 2.3% of the patients, while in the literature percentages between 5.9 and 7.8% have been reported [Citation10,Citation12,Citation26]. Patients <50 years demonstrated only a few differences compared to patients between 50 and 59 years in our study. Nonetheless, OS of patients with early-onset PDAC was better compared to patients between 50 and 59 years (8.5 vs. 6.5 months), which seems related to a higher rate of anticancer treatment in patients younger than 50 years (79 vs. 64%).
Patients <60 years diagnosed in academic hospitals had a less advanced stage of disease compared to patients in non-academic hospitals. This might be explained by earlier detection in patient cohorts being under surveillance in academic hospitals and less delay in diagnosis (‘lead time’), e.g., in patients with a history of cancer. Overall, general practitioners may refer young symptomatic patients to an academic hospital more often, while older patients may prefer a hospital nearby where they are already known for other diseases. Such practices will not likely be related to the stage of disease at diagnosis. Better OS in academic hospital of diagnosis seemed to be explained by the less advanced stage of disease in these patients, as this association did not persist after adjusting for the disease stage.
This study has several limitations. First, the NCR registry might be incomplete for older patients as previously described [Citation27]. This might cause an overestimation of the survival in older patients, as unregistered patients were more often older and had poorer prognosis. Therefore, real survival differences between young and older patients might be larger than observed in this study. Second, it was not possible to compare patients by the commonly used disease stages for PDAC [(borderline) resectable, locally-advanced, and metastatic], as this was not registered in the NCR. Therefore, we stratified patients by treatment groups instead. Third, limitations inherent to the retrospective nature of the study could not be avoided, such as missing data and confounding by unknown variables. For example, family history and BRCA status were not registered in the NCR, hereby we could not investigate the association between familial predisposition and PDAC at a young age.
In conclusion, patients <60 years were more often male, had less comorbidities, had less stage I at diagnosis, and received more anticancer treatment compared to older patients. OS was better in PDAC patients <60 years, however, this difference did not persist when stratified for treatment strategy, except for treated patients with stage IV disease.
No preregistration exists for the reported studies reported in this article.
Data availability statement
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data is not available.
Supplemental Material
Download MS Word (16.7 KB)Supplemental Material
Download MS Word (15.2 KB)Supplemental Material
Download MS Word (14.8 KB)Supplemental Material
Download PDF (142 KB)Supplemental Material
Download MS Word (29.5 KB)Acknowledgements
The authors would like to thank the registration personnel from the Netherland Comprehensive Cancer Organization (IKNL) for the collection of the NCR data in all hospitals in the Netherlands.
Disclosure statement
Judith de Vos-Geelen has served as a consultant for Amgen, AstraZeneca, MSD, Pierre Fabre, and Servier, and has received institutional research funding from Servier. All outside the submitted work. Other authors have no COI to report.
References
- Latenstein AEJ, van der Geest LGM, Bonsing BA, et al. Nationwide trends in incidence, treatment and survival of pancreatic ductal adenocarcinoma. Eur J Cancer. 2020;125:83–93.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA A Cancer J Clin. 2020;70(1):7–30.
- Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer. 2002;2(12):897–909.
- Carioli G, Malvezzi M, Bertuccio P, et al. European cancer mortality predictions for the year 2021 with focus on pancreatic and female lung cancer. Ann Oncol. 2021;32(4):478–487.
- Saad AM, Turk T, Al-Husseini MJ, et al. Trends in pancreatic adenocarcinoma incidence and mortality in the United States in the last four decades; a SEER-based study. BMC Cancer. 2018;18(1):688.
- Raimondi S, Maisonneuve P, Lohr JM, et al. Early onset pancreatic cancer: evidence of a major role for smoking and genetic factors. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1894–1897.
- Latenstein AEJ, Mackay TM, Creemers GJ, et al. Implementation of contemporary chemotherapy for patients with metastatic pancreatic ductal adenocarcinoma: a population-based analysis. Acta Oncol. 2020;59(6):705–712.
- Brada LJH, Walma MS, van Dam RM, et al. The treatment and survival of elderly patients with locally advanced pancreatic cancer: a post-hoc analysis of a multicenter registry. Pancreatology. 2021;21(1):163–169.
- van der Geest LG, Besselink MG, van Gestel YR, et al. Pancreatic cancer surgery in elderly patients: Balancing between short-term harm and long-term benefit. A population-based study in The Netherlands. Acta Oncol. 2016;55(3):278–285.
- Ordonez JE, Hester CA, Zhu H, et al. Clinicopathologic features and outcomes of early-onset pancreatic adenocarcinoma in the United States. Ann Surg Oncol. 2020;27(6):1997–2006.
- Eguchi H, Yamaue H, Unno M, et al. Clinicopathological characteristics of young patients with pancreatic cancer: an analysis of data from pancreatic cancer registry of Japan pancreas society. Pancreas. 2016;45(10):1411–1417.
- Alese OB, Jiang R, Shaib W, et al. Young adults with pancreatic cancer: national trends in treatment and outcomes. Pancreas. 2020;49(3):341–354.
- Goksu SY, Kazmi SMA, Sanford NN, et al. Distinct clinical characteristics of young-onset pancreatic cancer patients. J Clin Oncol. 2020;38(4_suppl):674.
- Ben-Aharon I, Elkabets M, Pelossof R, et al. Genomic landscape of pancreatic adenocarcinoma in younger versus older patients: does age matter? Clin Cancer Res. 2019;25(7):2185–2193.
- Heining C, Horak P, Uhrig S, et al. NRG1 fusions in KRAS wild-type pancreatic cancer. Cancer Discov. 2018;8(9):1087–1095.
- Singhi AD, George B, Greenbowe JR, et al. Real-time targeted genome profile analysis of pancreatic ductal adenocarcinomas identifies genetic alterations that might be targeted with existing drugs or used as biomarkers. Gastroenterology. 2019;156(8):2242–2253.e2244.
- Kim SY, Weinberg L, Christophi C, et al. The outcomes of pancreaticoduodenectomy in patients aged 80 or older: a systematic review and meta-analysis. HPB. 2017;19(6):475–482.
- Tamirisa NP, Parmar AD, Vargas GM, et al. Relative contributions of complications and failure to rescue on mortality in older patients undergoing pancreatectomy. Ann Surg. 2016;263(2):385–391.
- Strijker M, Mackay TM, Bonsing BA, et al. Establishing and coordinating a nationwide multidisciplinary study group: Lessons learned by the Dutch Pancreatic Cancer Group. Ann Surg. 2020;271(4):e102–e104.
- von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457.
- Varghese AM, Singh I, Singh R, et al. Early-onset pancreas cancer: clinical descriptors, genomics, and outcomes. J Natl Cancer Inst. 2021;113(9):1194–1202.
- Yeo H, Betel D, Abelson JS, et al. Early-onset colorectal cancer is distinct from traditional colorectal cancer. Clin Colorectal Cancer. 2017;16(4):293–299.e296.
- Fairley TL, Cardinez CJ, Martin J, et al. Colorectal cancer in U.S. adults younger than 50 years of age, 1998–2001. Cancer. 2006;107(5 Suppl):1153–1161.
- Murphy KM, Brune KA, Griffin C, et al. Evaluation of candidate genes MAP2K4, MADH4, ACVR1B, and BRCA2 in familial pancreatic cancer: deleterious BRCA2 mutations in 17%. Cancer Res. 2002;62(13):3789–3793.
- Zhen DB, Rabe KG, Gallinger S, et al. BRCA1, BRCA2, PALB2, and CDKN2A mutations in familial pancreatic cancer: a PACGENE study. Genet Med. 2015;17(7):569–577.
- Ansari D, Althini C, Ohlsson H, et al. Early-onset pancreatic cancer: a population-based study using the SEER registry. Langenbecks Arch Surg. 2019;404(5):565–571.
- Fest J, Ruiter R, van Rooij FJ, et al. Underestimation of pancreatic cancer in the national cancer registry – reconsidering the incidence and survival rates. Eur J Cancer. 2017;72:186–191.