Abstract
Background
There is very limited data available on how most breast cancer recurrences, either distant metastases or locoregional recurrences (LRR), are actually discovered in routine clinical practice.
Patients and methods
From a prospective cohort of 621 women diagnosed and treated for early invasive breast cancer between 2003 and 2013, we analysed the patients who were later diagnosed with distant metastases (n = 61) and the patients who had locoregional recurrences (LRR; n = 34). The patients had routine control visits for up to 10 years from initial diagnosis, with annual clinical visits, mammography, blood count, plasma creatinine and liver function tests.
Results
Most distant metastases (n = 38, 62%) were found when a patient contacted health care services because of a symptom; only ten (16%) were detected at pre-planned control visits. The most common first sign or symptom of metastasis was pain (n = 23, 38%). Pain as the first indicator of metastasis indicated a lower survival in metastatic disease (hazard ratio 4.40; 95% confidence interval 1.77–10.94; p = 0.001). How relapse was detected or whether patient was symptomatic did not affect overall survival (OS) of patients with distant metastases. LRRs were mostly found at pre-planned control visits (n = 14, 41%). Abnormalities in routine laboratory tests did not lead to any detection of recurrence.
Discussion
In this prospective, contemporary, real-world study, the vast majority of both distant metastases and LRRs were detected outside the pre-planned control visits. These results highlight the importance of finding ways to lower the threshold for contacting the surveillance unit, rather than frequent routine controls.
Introduction
After curative treatment, breast cancer survivors need follow-up in health care in order to monitor possible adverse effects of treatment, offer support in cancer survivorship and detect possible recurrence of the disease. However, there is no clear consensus on how the follow-up of patients after the treatment of local breast cancer should be organised [Citation1,Citation2].
Most of the current breast cancer surveillance guidelines recommend clinical examination and mammography for asymptomatic patients and further examinations only according to patients’ possible symptoms [Citation3,Citation4]. Extensive imaging or laboratory tests are not a part of routine follow-up for asymptomatic patients, as they increase costs and the rate of false-positive findings [Citation1,Citation5]. Although intensive follow-up has been shown to detect distant recurrences earlier, it is not yet proven to improve true-positive rates of distant metastases or survival [Citation1,Citation6,Citation7]. In contrast, earlier finding of locoregional recurrences (LRR) also leads to an improved prognosis [Citation8].
In the era of modern treatment, there is a very limited amount of real-world information available on whether most breast cancer recurrences, either LRRs or distant metastases, are actually found during routine control visits, coincidentally or due to extra contact with health care after a recurrence-related symptom, and whether the means of metastasis detection has a prognostic role. In this study, we prospectively followed a large Finnish University Hospital–based dataset of early breast cancer patients in order to assess these clinically important issues, and also to determine the most common signs or symptoms of recurrence and whether these factors affected prognosis.
Materials and methods
Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were used in conducting the study (Supplementary file 1). The data originated from a prospective cohort of 621 women diagnosed and treated for early invasive breast cancer, that is, stages I–III in Oulu University Hospital between 2003 and 2013 (). Patients with prior breast cancer or de novo metastatic breast cancer were excluded from the cohort. The cohort collected prospectively all available patients meeting these entry criteria, who had given the informed consent and were treated at the centre during the study period. Approximately 250 new breast cancer patients were annually diagnosed and treated at the centre at the time. In this study, we focussed on the 61 patients who were later diagnosed with distant metastases and the 34 patients who had LRR before January 2019. The final dataset consisted of 89 patients since six patients developed distant metastases after LRR and were included in both of the final cohorts (). The data were collected from medical records and there were no dropouts or lost of follow-up. LRRs were defined as breast cancer recurrences in a resection scar, ipsilateral or contralateral breast or axilla, while distant metastases were clinically or pathologically verified breast cancer metastases outside these areas.
Figure 1. The final dataset consisted of 89 patients divided into two cohorts. Six patients had both distant metastases and locoregional recurrence and were included in both cohorts.
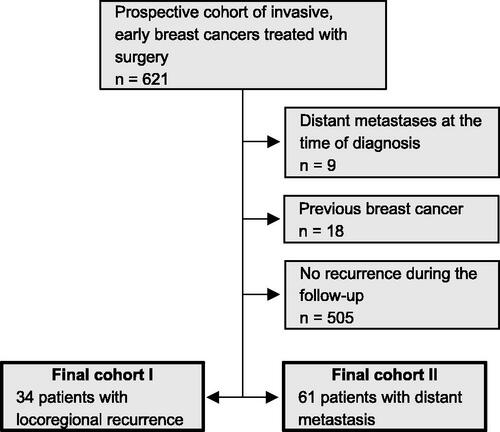
Table 1. Primary characteristics of patients with a locoregional recurrence or distant metastases.
The current WHO guidelines were used in histopathological evaluation and stages were assessed using TNM classification. Immunohistochemical analyses to determine oestrogen receptor (ER), progesterone receptor (PR) and Ki-67 expression were carried out as previously described [Citation9]. Human epidermal growth factor 2 (HER2) expression was studied using immunohistochemistry and positive results were confirmed by chromogenic in situ hybridisation [Citation10].
Of the 89 patients with known breast cancer relapse, we classified the way the relapse was found as follows: (1) a patient noticed a relapse-related symptom and contacted health care between pre-planned control visits; (2) by a health care professional during a pre-planned breast cancer control visit; (3) as a coincidental finding in non-breast-cancer-related visits or in breast cancer screenings. We also divided the first indicators, that is, the first signs or symptoms, of relapse into five categories: (1) pain; (2) respiratory symptoms; (3) decrease in general condition; (4) palpable or visible lesion detected by the patient; (5) imaging or laboratory abnormalities or a finding detected in a clinical examination.
Surveillance scheme
All patients were followed within the health care system for up to 10 years. The annual controls included clinical examination, mammography with possible complementary breast and/or axilla ultrasound, and laboratory tests, including complete blood count (without leukocyte differential count), plasma creatinine and liver function tests. Surveillance was carried out in primary health care and/or in the Oulu University Hospital depending on the evaluated recurrence risk of the patient. The follow-up protocol was the same in primary health care and in the University Hospital.
Low-risk patients with breast cancers of T1N0, grade I and >59% oestrogen receptor expression was followed in primary health care. Medium-risk patients (those not meeting low-risk or high-risk criteria) were followed in the University Hospital for the first two years, and after that in primary health care for up to 10 years. High-risk patients with four or more metastatic axillary lymph nodes, HER2-positive cancer or triple-negative breast cancer were followed in the University Hospital and primary health care in alternate years for the first five years, and then in primary health care for up to 10 years. Patients were encouraged to contact their surveillance unit between control visits if cancer-related symptoms occurred. Patients under University Hospital surveillance could contact a trained breast cancer nurse in case of suspicious symptoms.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics software (v. 25.0.0.0 and v. 27.0.0.0) for Mac (IBM Corporation, Armonk, NY, USA). Survival was analysed using Kaplan–Meier curves and a log-rank test. Distant disease-free survival (DDFS) was defined as the time between surgery and metastasis diagnosis and local recurrence-free survival (RFS) as the time between surgery and LRR diagnosis. Overall survival (OS) was calculated from the date of surgery to the time of death or the end of the follow-up. Survival in metastatic disease was calculated from the date when metastasis was first confirmed in imaging to the time of death or the end of follow-up. Multivariate analysis was conducted using Cox regression analysis with the strongest traditional prognostic factors, nodal status, grade and ER expression as co-variates. These co-variates were divided into two-classed variables: N0 or N1-3, grades 1–2 or 3 and ER positive or negative. Associations were calculated with 2-sided chi-square tests. P-values less than 0.05 were considered significant in all analyses. Missing data were excluded from the analyses.
Ethical considerations
The patients provided their written informed consent to participate in the study. The study was approved by the Local Ethics Committee of the Ostrobothnia Hospital District (114/2011). All studies were conducted in accordance with the principles of the Declaration of Helsinki and the guidelines for Good Clinical Practice.
Results
Clinicopathological features and general survival
Patient and treatment characteristics are displayed in and primary breast cancer characteristics in . Median follow-up time for all patients was 98 months: 72 months for patients with metastases and 136 months for patients with LRR. Fifty of 61 patients with distant metastases died of breast cancer by the end of the follow-up, 10 patients were alive with breast cancer and one was radiologically disease-free. Median OS for patients with distant metastases was 72 months, DDFS 39 months and median survival in metastatic disease 21 months.
Table 2. Primary tumour characteristics of patients with a locoregional recurrence or distant metastases.
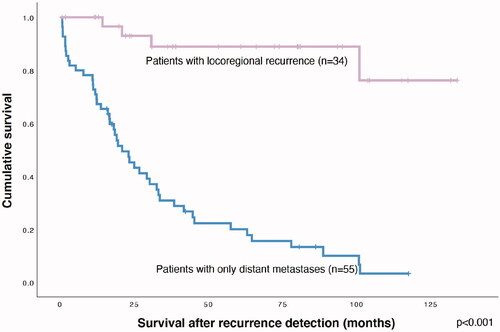
Six out of 34 patients with LRR were subsequently diagnosed with distant metastases and three of them died during the follow-up period. One patient with LRR died of unknown causes and the rest of the patients were alive, either with (n = 4) or without (n = 26) cancer. Median OS for patients with LRR was 135 months, RFS 73 months and median survival after LRR 46 months. Survival after locoregional recurrence of patients with LRR and survival in metastatic disease of patients with only distant metastasis are displayed in .
Distant metastasis detection and the first indicators of distant metastases
The majority of distant metastases were detected outside the control visits (). Most (n = 38, 62%) of distant metastases were found when the patient contacted health care because of a symptom and 12 (20%) distant metastases were coincidental findings in other medical examinations. Only ten (16%) distant metastases were detected at pre-planned control visits.
Figure 3. Pie charts showing how distant metastases (A–B) and locoregional recurrences (C–D) were diagnosed.
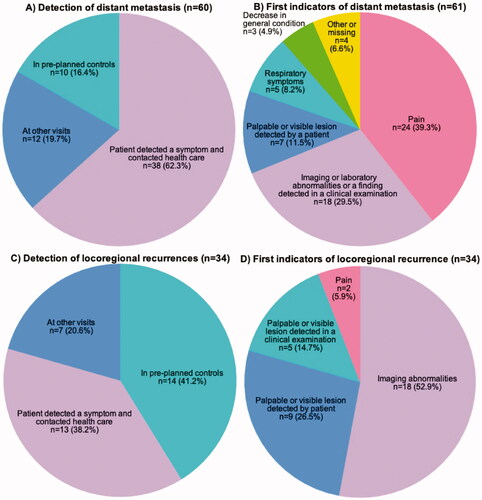
The first indicators of distant metastases were pain (n = 23, 38%), abnormalities in imaging (ultrasound, computed tomography (CT) scans, native X-ray, magnetic resonance imaging), laboratory tests or clinical examination (n = 18, 30%), palpable or visible lesion detected by the patient (n = 7, 12%), respiratory symptoms (n = 5, 8%) and decrease in general condition (n = 3, 5%), with five cases with missing information (8%). Overall, 48 (79%) patients with distant metastasis had symptoms at the time of diagnosis of distant metastasis, pain being the most common (n = 27, 44%). Distant metastases were confirmed with imaging (n = 20), biopsy or cytology (n = 16) or both (n = 15). Imaging modalities used were CT scans, magnetic resonance imaging and ultrasound combined with biopsy.
Locoregional recurrence detection and the first indicators of locoregional recurrences
LRRs were found mostly at pre-planned control visits (14 cases, 41%). The rest of the LRRs were detected after the patient contacted health care because of a relapse-related symptom (n = 13, 38%) or as coincidental findings or in screening mammograms (n = 7, 21%). The first indicator of LRR was breast imaging in 18 cases (53%). Mammography findings led to suspected LRR in 14 cases (41%), of which four were detected in mammograms via the national screening program. Local recurrence was suspected based on breast ultrasound in three cases (9%) and on breast MRI in one case (3%). Other first indicators were palpable or visible lesions detected by the patient in nine cases (27%), palpable or visible lesions detected in clinical examinations in five cases (15%) and pain in two cases (6%). Overall, 16 patients (47%) were symptomatic at the time of LRR detection. LRR were confirmed with biopsy or mastectomy (n = 22), mammogram (n = 3) or both (n = 6).
No distant metastases or LRR were detected by laboratory abnormalities in routine laboratory tests.
Factors affecting survival of patients with distant metastases
The first indicator of distant metastasis affected the length of survival in metastatic disease (p < 0.0010), although the low number of cases in some subgroups potentially limits the reliability of these analyses in these subgroups (). Multivariate analysis showed that those with pain as the first indicator of metastasis had worse survival in metastatic disease than those whose metastases were diagnosed due to imaging or laboratory abnormalities, or in a clinical examination (hazard ratio [HR] 4.40; 95% CI 1.77–10.94; p = 0.0014; nodal status HR 1.44; 95% CI 0.55–3.77; p = 0.45; grade HR 4.95; 95% CI 1.98–12.41; p = 0.00064; ER expression HR 1.16; 95% CI 0.51–2.62; p = 0.73). The first indicators of metastasis did not affect OS (p = 0.19).
Figure 4. Kaplan–Meier curves showing the effect of the first sign or symptom of metastasis (A) or the presence of symptoms (B) on survival in metastatic disease. (C) shows the effect of metastasis detection on survival for metastatic disease and (D) the effect of symptoms on survival after locoregional recurrence.
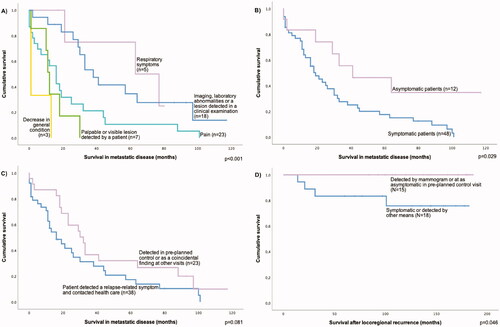
Presence of symptoms at the time of diagnosis of distant metastasis was linked to poorer survival in metastatic disease (p = 0.029). The result was confirmed in multivariate analysis (HR 2.48; 95% CI 1.07–5.76; p = 0.034, nodal status HR 1.82; 95% CI 0.88–3.74; p = 0.11, grade HR 0.53; 95% CI 0.27–1.00; p = 0.048, ER expression HR 2.32; 95% CI 1.15–4.67; p = 0.018). Asymptomatic patients had non-significantly shorter DDFS (p = 0.067) and OS was similar between groups.
Patients, whose metastasis was detected after they contacted health care because of a relapse-related symptom had longer DDFS than those, whose metastasis was detected in pre-planned control or other visits (p = 0.049). The result was confirmed in multivariate analysis (HR 1.77; 95% CI 1.01–3.10; p = 0.045, nodal status HR 0.83; 95% CI 0.43–1.60; p = 0.58; grade HR 1.54; 95% CI 0.82–2.89; p = 0.18; ER expression HR 3.06; 95% CI 1.39–6.73; p = 0.0053). Survival in metastatic disease was poorer in the first group, though the difference was not statistically significant (p = 0.081). OS was similar between groups.
Factors affecting survival of patients with LRR
Survival after LRR was longer if the LRR was detected asymptomatically at pre-planned control visit or was detected by mammogram than if the LRR was detected otherwise or was symptomatic (p = 0.046). However, this difference was significant only in univariate analysis.
Discussion
In this prospective data collected from a Finnish University Hospital, the majority of both distant metastases and LRRs were detected outside of the pre-planned annual control visits. Pain was the most common first indicator of distant metastasis, while imaging revealed the most LRRs. Almost 80% of the patients with distant metastasis and nearly half of those with LRRs were symptomatic when the relapse was diagnosed. The patients with symptomatic metastases at the time of the metastasis diagnosis had shortened survival in metastatic disease, with no change in OS.
Despite annual routine controls for up to 10 years, only one-sixth of the distant metastases were detected at pre-planned control visits. The majority (62%) of distant metastases were detected at interval visits when patients contacted health care due to a metastasis-related symptom. Previous studies reported 26% [Citation4,Citation6] and 29% [Citation4,Citation6] of distant metastases discovered at pre-planned control visits. However, in neither of the studies were coincidental findings separately reported [Citation4,Citation6], whereas 20% of distant metastases were coincidental findings in our study. Both previous studies also reflect treatments and patient characteristics typical of the 1970s and 1980 s. In these studies, the patients were also reviewed by an oncologist 2–4 times a year for the first five years and annually thereafter [Citation4,Citation6], which may explain their slightly higher proportion of distant metastases discovered at routine visits. Another old study, with a similarly intense follow-up, in which both local and distant recurrences were pooled together, 56% of the recurrences were discovered in routine visits [Citation11].
In our study, almost half (41%) of LRRs were found in annual routine control visits and an additional 11% via the national screening mammogram program. A meta-analysis of studies between 1982 and 2002 [12] found that a total of 59% (range 27–91%) of LRRs were detected at control visits. While the patient material of the studies included in the meta-analysis was generally comparable with that of the current study, the follow-up times were shorter throughout (medians 18–75 months) than in our study and patients in most of the studies included in the meta-analysis were examined more frequently than in our surveillance protocol [Citation12].
Considering all 89 patients of this study, symptoms were the first indicator of any relapse in 37% of cases. This is well in line with previous research, which consists of four retrospective studies in which symptoms were reported as the first indicator of any breast cancer relapse in 24–58% of cases [Citation13–16]. Visible or palpable lesions detected by patient or physician was reported as the first indicator of 19–38% of all relapses in previous studies [Citation14,Citation16], which is also concordant with our results (23%). In studies published after 2010, proportions of imaging and laboratory abnormalities (4–45% and 12–20%, respectively) as the first indicator of any relapse are larger than in older studies (10% altogether) [Citation14–17] Our results are thus in line with the more recent research showing imaging abnormalities as the first indicator of any relapse in 36% of cases. According to our knowledge, no previous prospective research in this field exists. Abnormalities in routine laboratory tests (blood count, plasma creatinine liver function tests) did not lead to detection of any relapse in our study. Although it would have been interesting to know the false-positive rates of laboratory tests, they were not in the scope of this study.
The first indicator in 52% of LRRs was abnormalities in imaging, followed by palpable or visible lesion detected by the patient (27%), findings in clinical examination (15%), and pain (4% of cases). In a somewhat old, but still the latest, meta-analysis by Montgomery et al., 25% of LRRs were detected by a mammography, 30% in clinical examination, 41% by patients, and 4% were coincidental findings [Citation18] Montgomery et al. also reported that possibly treatable LRRs were detected by patients in 30-40% of cases and the rest mammographically in 15% or 40% and by routine clinical examination in 46% or 15% of cases, depending whether the study was published before or after 2000, respectively [Citation18].
Symptoms were the first indicators of distant metastases in 57% of cases, while in the recent study of Itani et al., 78% of breast cancer distant metastases were diagnosed due to symptoms [Citation19]. The proportion of symptomatic patients at the time of breast cancer relapse diagnosis has been in the range of 47–58% in studies where local and distant relapses have been pooled together [Citation15–17]. Ogawa et al. reported in 2013 that only 24% of patients were symptomatic at the time of detection of breast cancer relapse of any site with a follow-up schedule of 6–12-month intervals [Citation15]. Patients with distant metastases are more frequently symptomatic (70–96%) than those with only LRRs (30–56%) [Citation4,Citation6,Citation20]. Our results are in line with the aforementioned studies, as 79% of the patients with distant metastases and 47% of the patients with LRRs were symptomatic at the time of diagnosis, even though our patients had rarer routine visits than in most of the previous studies.
Symptomatic patients, especially if the symptom was pain, had worse prognoses in metastatic disease than asymptomatic patients. Importantly, the presence of the symptoms at the time of the metastatic disease diagnosis did not have effect on OS. Even though the number of cases was limited in this analysis, an HR of 4.40, including the most powerful traditional prognostic factors in the analysis, suggests that there is a real effect. The literature in this field is very scarce, but a recent retrospective study of 371 breast cancer patients did not find an association between the presence of any symptoms at the time of metastatic disease diagnosis and prognosis [Citation19]. However, in a study of 61 distant relapses, Ogawa et al. found that when metastasis was detected by imaging or increased tumour markers, post-relapse survival and OS were significantly better than those detected based on symptoms or clinical examination [Citation14]. In our study, there was a trend of prolonged survival in metastatic disease when metastases were detected at control visits or as coincidental findings. In itself, this could imply that early detection of distant metastases could improve survival odds, but since overall survival was not affected, the difference in survival in metastatic disease could be partly explained by lead time and length time biases [Citation21]. Early detection of breast cancer relapse has been shown to give survival benefit, but only in studies where distant and locoregional relapses were analysed together. The benefit was also clearer when only LRRs were analysed [Citation8].
The overall five-year survival after LRRs was 91% and ten-year survival 87%. These relatively high percentages may reflect not only effective postrecurrence treatments, but also a good prognosis of in-breast recurrences, which altogether covered 77% of all LRR in this study. Only 18% of patients with LRRs had synchronous distant metastases or were later diagnosed with distant metastases, whereas the proportion was reported to be 57% in 1985 [Citation4] and 29% in 1998 [Citation6]. In a meta-analysis of studies between 1987 and 2004, the detection of isolated LRRs at an asymptomatic phase, at control visits or by mammogram was shown to confer significant survival benefit [Citation8], which was also suggested by a univariate, but not by a multivariate, analysis in this study.
The strengths of this study include a prospective setting and real-world data that is contemporary yet with a long follow-up. As the patients in this study were treated with modern methods, the results are valuable in the evaluation of current practices. However, the number of patients with recurrences, especially with LRRs, is low considering multifactorial correlations and separate analyses on breast cancer subtypes, and thus the results are mostly descriptive in places. The median survival with metastatic breast cancer was 23 months in this study, while previous estimates have been around 36 months [Citation22]. The lack of de novo metastatic breast cancers in our study may explain this discrepancy to some extent. We were unable to determine how the local follow-up guidelines were actually followed, especially in primary health care, but based on our clinical experience, they are typically well adhered-to.
Conclusion
We conclude that in this prospective, contemporary, real-world study, the majority of both distant metastases and LRRs were detected outside of pre-planned control visits. Pain was the most common first indicator of distant metastasis and its presence also indicated a dismal survival prognosis in metastatic disease, with no impact on OS. However, LRRs were still often detected at pre-planned control visits especially by routine mammograms. Although the surveillance cannot be seen solely from the somatic perspective [Citation3,Citation23], these results highlight the importance of finding ways to lower the threshold for contacting the surveillance unit in addition to regular routine controls. For example, web-based surveillance has been shown to have good adherence, and to improve the reporting of symptoms during radiotherapy [Citation24] and to improve quality of life while decreasing the number of emergency visits and hospitalisations in metastatic breast cancer during chemotherapy [Citation25].
Supplemental Material
Download MS Word (33.3 KB)Disclosure statement
There are no potential conflicts of interest
Data availability statement
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data is not available.
Additional information
Funding
References
- Rojas MPMP, Telaro E, Moschetti I, et al. Follow-up strategies for women treated for early breast cancer. Cochrane Database Syst Rev. 2000;(4):CD001768.
- Cruickshank S, Barber M. Breast cancer follow-up after a primary diagnosis: a confused picture. Breast. 2019;46:97–100.
- Cardoso F, Kyriakides S, Ohno S, et al.; ESMO Guidelines Committee. Electronic address: [email protected]. Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(8):1194–1220.
- Dewar JA, Kerr GR. Value of routine follow up of women treated for early carcinoma of the breast. Br Med J. 1985;291(6507):1464–1467.
- Louie RJ, Tonneson JE, Gowarty M, et al. Complete blood counts, liver function tests, and chest x-rays as routine screening in early-stage breast cancer: value added or just cost? Breast Cancer Res Treat. 2015;154(1):99–103.
- Loong S, Wilkins M, Bliss JM, et al. The effectiveness of the routine clinic visit in the follow-up of breast cancer patients: Analysis of a defined patient cohort. Clin Oncol (R Coll Radiol). 1998;10(2):103–106.
- Kokko R, Hakama M, Holli K. Follow-up cost of breast cancer patients with localized disease after primary treatment: a randomized trial. Breast Cancer Res Treat. 2005;93(3):255–260.
- Lu WL, Jansen L, Post WJ, et al. Impact on survival of early detection of isolated breast recurrences after the primary treatment for breast cancer: a meta-analysis. Breast Cancer Res Treat. 2009;114(3):403–412.
- Karihtala P, Mantyniemi A, Kang SW, et al. Peroxiredoxins in breast carcinoma. Clin Cancer Res. 2003;9(9):3418–3424.
- Isola J, Tanner M, Forsyth A, et al. Interlaboratory comparison of HER-2 oncogene amplification as detected by chromogenic and fluorescence in situ hybridization. Clin Cancer Res. 2004;10(14):4793–4798.
- Holli K, Hakama M. Effectiveness of routine and spontaneous follow-up visits for breast cancer. Eur J Cancer Clin Oncol. 1989;25(2):251–254.
- de Bock GH, Bonnema J, van der Hage J, et al. Effectiveness of routine visits and routine tests in detecting isolated locoregional recurrences after treatment for early-stage invasive breast cancer: a Meta-analysis and systematic review. J Clin Oncol. 2004;22(19):4010–4018.
- Pandya KJ, McFadden ET, Kalish LA, et al. A retrospective study of earliest indicators of recurrence in patients on Eastern cooperative oncology group adjuvant chemotherapy trials for breast cancer. A preliminary report. Cancer. 1985;55(1):202–205.
- Ogawa Y, Ikeda K, Izumi T, et al. First indicators of relapse in breast cancer: evaluation of the follow-up program at our hospital. Int J Clin Oncol. 2013;18(3):447–453.
- Pivot X, Asmar L, Hortobagyi GN, et al. A retrospective study of first indicators of breast cancer recurrence. Oncology. 2000;58(3):185–190.
- Viot J, Bachour M, Meurisse A, et al. Follow-up of patients with localized breast cancer and first indicators of advanced breast cancer recurrence: a retrospective study. Breast. 2017;34:53–57.
- Schapira DV. Breast cancer surveillance-a cost-effective strategy. Breast Cancer Res Treat. 1993;25(2):107–111.
- Montgomery DA, Krupa K, Cooke TG. Follow-up in breast cancer: does routine clinical examination improve outcome? A systematic review of the literature. Br J Cancer. 2007;97(12):1632–1641.
- Itani N, Grogan N, Mott S, et al. Metastatic presentations of previously treated early-stage breast cancer patients and association with survival. Clin Breast Cancer. 2020;20(3):209–214.
- Perrone MA, Musolino A, Michiara M, et al. Early detection of recurrences in the follow-up of primary breast cancer in an asymptomatic or symptomatic phase. Tumori. 2004;90(3):276–279.
- Tomin R, Donegan WL. Screening for recurrent breast cancer-its effectiveness and prognostic value. J Clin Oncol. 1987;5(1):62–67.
- Cardoso F, Senkus E, Costa A, et al. 4th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 4)†. Ann Oncol. 2018;29(8):1634–1657.
- Cheung WY, Neville BA, Earle CC. Associations among cancer survivorship discussions, patient and physician expectations, and receipt of follow-up care. J Clin Oncol. 2010;28(15):2577–2583.
- Takala L, Kuusinen TE, Skyttä T, et al. Electronic patient-reported outcomes during breast cancer adjuvant radiotherapy. Clin Breast Cancer. 2021;21(3):e252–e270.
- Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557–565.