Abstract
Background
It is uncertain whether centralization of gastrectomy to fewer surgeons and larger centers improves survival in gastric adenocarcinoma in Western populations. The aim of this study was to examine if higher annual surgeon or hospital volumes of gastrectomy increase gastric adenocarcinoma survival in a population-based Swedish cohort.
Methods
This study included almost all patients who underwent curatively intended gastrectomy for gastric adenocarcinoma in Sweden between 2006 and 2015 with follow-up throughout 2020. Data were collected from medical records and national registries. Annual surgeon and hospital volumes of gastrectomies were analyzed by categorization into four equal-sized groups and as continuous variables. The outcomes were 5-year all-cause mortality (main) and 5-year disease-specific mortality. Cox regression produced hazard ratios (HR) with 95% confidence intervals (95% CI), adjusted for sex, age, education, comorbidity, pathological tumor stage, pre-operative therapy, calendar period, and mutually for hospital or surgeon volume.
Results
The study included 1774 patients. Higher annual surgeon volume did not decrease the risk of 5-year all-cause mortality when comparing the highest and lowest quartiles (HR = 1.07, 95% CI 0.86–1.34) or when analyzed as a continuous variable (HR = 1.03, 95% 1.00–1.06). Higher annual hospital volume did not significantly decrease the risk of 5-year all-cause mortality (highest versus lowest quartiles: HR = 0.89, 95% CI 0.71–1.10; continuous variable: HR = 0.98, 95% CI 0.95–1.02). The results for 5-year disease-specific mortality were similar.
Conclusions
This study, mirroring routine clinical practices in an entire Western country, indicates that neither annual surgeon volume nor annual hospital volume of gastrectomy influences the long-term survival in gastric adenocarcinoma.
Introduction
Surgery is the mainstay treatment of gastric cancer (>95% adenocarcinoma), the third most common cause of cancer-related deaths worldwide [Citation1]. The incidence of gastric adenocarcinoma is declining, but because of aging populations, the absolute number of new cases is increasing [Citation2]. The reported 5-year survival rates vary, but most are in the range of 10–30% overall and 35–55% after surgery [Citation3–6]. Tumor stage, comorbidity, and fitness are the strongest known prognostic factors [Citation6,Citation7]. The main surgical alternatives are total or subtotal (distal) gastrectomy, depending on stage, location, growth pattern, and extent of the cancer [Citation8,Citation9], and wide resection margins and removal of regional lymph nodes are recommended [Citation10].
We have previously found that annual surgeon volume of esophagectomy is a strong prognostic factor in esophageal cancer, even after adjustment for hospital volume, while higher hospital volume was not [Citation11]. Less is known about the prognostic role of surgery volumes for gastric adenocarcinoma. Centralization of gastrectomy to fewer surgeons at larger centers may improve patient outcomes by gathering multidisciplinary expertise and experience, particularly in Western countries where the incidence of gastric adenocarcinoma is relatively low [Citation1]. Several studies have investigated short-term outcomes and found an association between higher surgeon and hospital volumes and a decreased risk of short short-term survival [Citation12–16]. Yet, most studies from Western countries comparing outcomes before and after centralization have shown little or no improvement in long-term survival [Citation17–19].
This study aimed to test two hypotheses in an unselected cohort of patients having undergone gastrectomy for gastric adenocarcinoma in a Western country: (1) 5-year survival is improved if the gastrectomy is performed by a higher-volume surgeon, independent of hospital volume and other confounders; and (2) 5-year survival is better if the gastrectomy is performed at a higher-volume hospital but is explained by surgeon volume.
Methods
Design
This was a Swedish nationwide population-based cohort study during the period 2006–2020. Data came from a source cohort entitled the “Swedish gastric cancer surgery study” (SWEGASS), which has recently been established and presented in detail elsewhere [Citation20]. In brief, SWEGASS includes all 2154 patients who have undergone total or subtotal gastrectomy for gastric adenocarcinoma in Sweden any time between 1 January 2006 and 31 December 2015. These patients have been followed up until 31 December 2020 for all-cause mortality and 31 December 2019 for disease-specific mortality. The patients included in SWEGASS were first identified as having a diagnosis of gastric adenocarcinoma in the Swedish Patient Registry or the Swedish Cancer Registry, combined with a procedure code representing total or subtotal gastrectomy in the Swedish Patient Registry. Thereafter, the eligible patients were identified after a thorough and uniform review of clinical data from medical records, including notes from multidisciplinary conferences, surgery, discharge, and a pathology review of the resected specimens. We used the STROBE guidelines for cohort studies when writing this report [Citation21]. The Regional Ethical Review Board in Stockholm approved the study.
Data collection
From the medical records, we collected the following clinical data of relevance for the present study: Surgical and oncological treatment, pathological tumor stage, resection margins, lymphadenectomy, and postoperative in-hospital complications (according to the well-validated Clavien-Dindo Classification). Additional data came from four well-established and well-validated national health data registries with almost 100% completeness: Swedish Patient Registry, which provided data on comorbidity (based on the well-validated Charlson Comorbidity Index); Swedish Cancer Registry, with data on all cancer diagnoses; Swedish Cause of Death Registry, which provided information on date and causes of death (also for deaths abroad); and The Longitudinal Integration Database for Health Insurance and Labor Market, for data on education.
Exposures
The main exposure was annual surgeon volume, defined as the number of gastrectomies for gastric adenocarcinoma per surgeon and calendar year. This was measured by identifying the name(s) of the operating surgeon(s) from the surgical charts for every included patient. Each gastrectomy was assigned to the most experienced surgeon whenever more than one surgeon conducted the resection, i.e., the surgeon with the highest annual volume was considered the primary surgeon. The secondary exposure was annual hospital volume, defined as the number of gastrectomies for gastric adenocarcinoma per hospital and calendar year. To compensate for fluctuations over time, a mean annual volume was calculated for both exposures, where from the year 2009 onwards, annual volume was defined as the volume of the same year and the three previous years divided by four. For the years 2006–2008, when data from the previous three years were not available, we used the information for the years or year available divided by the number of years. For annual surgeon volume, the annual numbers of gastrectomies among all 273 primary surgeons were analyzed in three ways: (1) categorized into four as equal-sized groups as possible (quartiles), corresponding to <2.3, 2.3–3.9, 4.0–5.7, and 5.8–14.8 gastrectomies per year, which was the main approach; (2) as a continuous variable; and (3) categorized into 14 groups (<1, 1-<2, 2-<3, etc., ending with ≥13). For annual hospital volume, the annual number of gastrectomies at any of the 60 relevant hospitals was also analyzed in three ways: (1) categorized into quartiles (<4.8, 4.8–8.4, 8.5–15.7, and 15.8–31.0), which was the main approach, (2) as a continuous variable, and (3) categorized into 10 groups (<3, 3-<6, 6-<9, etc., ending with ≥27).
Outcomes
The main outcome was 5-year all-cause mortality, i.e., any death independent of the cause within 5 years of gastrectomy. The secondary outcome was 5-year disease-specific mortality, i.e., when gastric cancer was recorded as a main or contributory cause of death within 5 years of gastrectomy in the Cause of Death Registry. We also planned to assess 90-day all-cause mortality as another secondary outcome, but there were too few deaths within this time frame for robust statistical analyses. Thus, 90-day mortality was only reported for descriptive purposes.
Statistical analysis
Crude survival probabilities were presented by Kaplan–Meier curves, stratified by quartiles of annual surgeon volume and hospital volume. Cox proportional hazards models provided hazard ratios (HR) with 95% confidence intervals (CI) for associations between the surgery volume exposures and mortality outcomes. Person-years of follow-up were calculated from the date of gastrectomy until death or end of follow-up, whichever occurred first. Three multivariable models were used: Model 1 adjusted for the covariates sex (categorized into male or female), age (continuous), education (≤9 years, 10–12 years, or ≥13 years), comorbidity (Charlson comorbidity index 0, 1, or ≥2), pathological tumor stage (0–I, II, III, or IV), pre-operative chemotherapy (yes or no), and calendar period (year 2006–2010 or 2011–2015). Model 2 adjusted for all variables in model 1 and further adjusted for hospital volume in analyses of surgeon volume and for surgeon volume in analyses of hospital volume. Model 3 was exploratory, i.e., used for assessing possible explanations for associations, and included all variables in model 2 and additionally surgical resection margins (R0 or R1/unclear), in-hospital complications (Clavien-Dindo 0–1 or ≥2), and the number of lymph nodes removed (three equal-sized groups, i.e., tertiles). Calendar period was included in the multivariable models to adjust for potential changes over time in pre-, peri- and post-operative surgical care. Some degree of centralization of surgery took place during the study period, therefore additional analysis without calendar period included in the models was conducted to examine if this affected the results. The proportional hazards assumption was evaluated using log–log survival plots and by calculating the correlations between Schoenfeld residuals for a particular covariate and ranking of individual failure time. The correlations were low, indicating that the proportional hazards assumption was met for all covariates.
To further evaluate whether potential associations between surgery volume and outcomes were modified by covariates, an interaction term was included in models 1 and 3, where HRs were derived within each stratum for surgeon volume (below or above median), hospital volume (below or above median), tumor stage (0–II or III/IV), and pre-operative therapy (yes or no). An analysis stratified the surgical approach by subtotal and total gastrectomy.
There were some missing data for the covariates education (2.0%), tumor stage (2.3%), pre-operative chemotherapy (0.2%), resection margins (5.6%), and lymph node yield (4.0%), and missing data occurred in at least one of these covariates in 12.3% of patients. This issue was managed by conducting both multiple imputation and a complete case analysis. In the multiple imputation analysis, 20 datasets were imputed and monotone logistic method in PROC MI was used with the assumption that missing data occurred at random (MAR) [Citation22]. Imputation was conducted separately for the two outcomes, and all covariates in model 3 were included. PROC MIANALYZE was used to combine the results from the analyses of the 20 datasets. Because the results from the multiple imputation and complete case analyses were similar and imputation was considered the best strategy to reduce bias, only findings from the multiple imputation analyses are presented in the tables.
An experienced biostatistician (FM) conducted the data management and statistical analyses according to a detailed and pre-defined study protocol and used the statistical software SAS/STAT Statistical Package, Version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Patients
A total of 2154 patients underwent gastrectomy for gastric adenocarcinoma during the study period. After exclusion of patients resected without curative intent (n = 298, 14%), patients who underwent minimally invasive surgery (n = 60, 2.8%), or had missing data on surgical approach (n = 22, 1.0%), 1774 patients remained for final analysis. Patient characteristics were rather equally distributed for most variables when stratified by quartiles of annual surgeon volume and hospital volume (). However, a larger lymph node yield and higher risk of complications was found in higher quartiles of surgeon and hospital volume, whereas frequencies of non-radical resection margins were similarly distributed across these quartiles ().
Table 1. Characteristics of 1774 study patients operated for gastric adenocarcinoma by quartile (Q) of annual surgeon and hospital volume, presented as numbers (%).
Absolute mortality rates
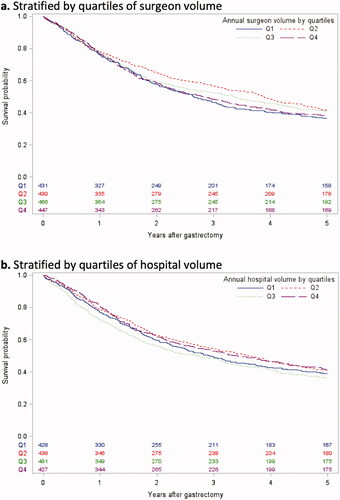
Kaplan–Meier curves did not show any major differences in overall survival within 5 years of gastrectomy comparing quartiles of annual surgeon volume or hospital volume (). The mortality rates were similar comparing patients operated by surgeons in the lowest versus highest volume quartile (5% versus 5% for 90-day mortality, 63% versus 62% for 5-year all-cause mortality, and 53% versus 55% for 5-year disease-specific mortality), as were the corresponding frequencies for hospital volume (5% versus 3%, 61% versus 59%, and 52% versus 48%).
Annual surgeon volume and risk of 5-year mortality
Higher quartile of annual surgeon volume did not decrease the risk of 5-year all-cause mortality in any of the three multivariable models (). When comparing the highest with the lowest quartile of annual surgeon volume, the HR was 1.04 (95% CI 0.87–1.24) in model 1, 1.07 (95% CI 0.86–1.34) in model 2, and 1.12 (95% CI 0.90–1.40) in model 3. When analyzed as a continuous variable, increasing surgeon volume did not show any decreased risk of 5-year all-cause mortality (HR 1.03, 95% 1.00–1.06). The results did not change when calendar period was omitted from all three models in the analysis (data not shown). The analysis with annual surgeon volume categorized into 14 groups did not show any trend of lower 5-year all-cause mortality with higher volume (). The analyses stratified by annual surgeon volume and annual hospital volume provided similar results to the unstratified analyses (). Stratification by tumor stage and pre-operative chemotherapy did not change the results (data not shown). The analysis with stratification by subtotal and total gastrectomy did not show any associations (data not shown). There were no statistically significant differences between annual surgeon volume or hospital volume in relation to risk of 5-year all-cause or disease-specific mortality ().
Table 2. Annual surgeon and hospital volume by quartile (Q) and risk of 5-year mortality after gastrectomy for gastric adenocarcinoma, expressed as hazard ratios (HR) with 95% confidence intervals (CI).
Table 3. Annual surgeon and hospital volume with 14 and 10 categories, respectively, and risk of 5-year all-cause mortality after gastrectomy for gastric adenocarcinoma, expressed as hazard ratios (HR) with 95% confidence intervals (CI).
Table 4. Annual surgeon and hospital volume and risk of 5-year mortality after gastrectomy for gastric adenocarcinoma, expressed as hazard ratios (HR) with 95% confidence intervals (CI), stratified by annual surgeon and hospital volume.
Annual hospital volume and risk of 5-year mortality
Higher quartiles of annual hospital volume were not associated with any statistically significantly decreased risk of 5-year all-cause mortality in any of the multivariable models, although most point estimates were slightly below 1.0 (). When comparing the highest with the lowest quartiles of hospital volume, the HR was 0.92 (95% CI 0.77–1.10) in model 1, 0.89 (95% CI 0.71–1.10) in model 2, and 0.91 (95% CI 0.72–1.13) in model 3. When hospital volume was analyzed as a continuous variable, higher volume did not decrease the risk of 5-year all-cause mortality (HR 0.98, 95% CI 0.95–1.02). The results did not change when calendar period was omitted from all three models in the analysis (data not shown). The analysis with annual hospital volume categorized into 10 groups did not reveal any trend between higher hospital volume and decreased 5-year all-cause mortality (). The analyses stratified by annual surgeon volume and hospital volume provided similar results to the unstratified analyses (). Stratification by tumor stage and pre-operative chemotherapy did not change the results (data not shown). Regarding 5-year disease-specific mortality, in models 1, 2, and 3 comparing the highest with the lowest annual hospital volume showed HRs of 0.86 (95% CI 0.70–1.05), 0.78 (95% CI 0.61–0.99), and 0.79 (95% CI 0.62–1.01), respectively (). In the analysis with stratification by type of resection (subtotal and total gastrectomy), hospital volume was not associated with any decreased risk of 5-year all-cause mortality. However, the highest quartile of hospital volume was associated with decreased HRs of 5-year disease-specific mortality after total gastrectomy (HR 0.70, 95% CI 0.52–0.93), but without any dose-response pattern (data not shown).
Discussion
The results of this study do not support the hypotheses that higher annual surgeon volume and higher hospital volume of gastrectomy are associated with better 5-year survival in patients with gastric adenocarcinoma.
A strength of this study is the population-based design with the inclusion of almost all patients who underwent curatively intended gastrectomy for gastric adenocarcinoma in an entire Western country during a 10-year period, mirroring clinical practice. The complete follow-up for up to 5 years is another advantage, which removed the otherwise common issue with losses to follow-up. The method of objective data assessment by external researchers (not self-reported by surgeons or hospitals) using prospectively recorded information from both medical records and well-established nationwide registries minimized information and selection bias. The data regarding the annual surgery volume (exposures) and 5-year mortality (outcomes) were highly accurate, and the collection of data on all known prognostic factors enabled adjustment for all key confounders, including mutual adjustment for surgeon and hospital volume. The restriction only to patients with adenocarcinoma should make the results more valid because other histological types of gastric malignancy may have different treatment and prognosis. Among limitations is the lack of information on other major surgical procedures that the surgeons and hospitals conducted during the study period, not least esophagectomies, which could have influenced the surgical proficiency. Another weakness is that the relatively low incidence of gastric adenocarcinoma in Sweden reduced the numbers of annual gastrectomies performed per surgeon and hospital. Therefore, the study could not examine potential associations with high-volume surgeons or hospitals from an international perspective, making the results less generalizable. However, the analyses of more exposure groups did not reveal any indications of associations even in the highest-volume categories. The statistical power was limited in some analyses, which introduced a risk of type II errors. Type I errors could also have occurred, e.g., from multiple testing, and might explain the very small number of statistically significant results, although we restricted the number of tests only to those included in the pre-defined study protocol.
Hospital and surgeon volume have previously been widely studied for several types of complex cancer surgery, but less so for gastrectomy for gastric cancer, and there is no consensus on the effect of volume on long-term survival for this cancer type. A difficulty with interpreting results from gastrectomy volume studies on gastric cancer mortality is the large differences in the definition of volume depending on where in the world the study is conducted, reflecting the wide geographical variation in incidence. A cohort study from the high-incidence country Taiwan including 6909 patients, found an association between higher annual surgeon volume and improved 5-year survival, but no association with hospital volume [Citation23]. On the other hand, a Japanese study analyzed the results from 521 patients included in either of two randomized clinical trials and found no association between annual surgeon volume and 5-year survival [Citation24]. A recent review found better survival after surgery for gastric cancer conducted at high-volume hospitals in four out of nine studies, while the remaining five studies displayed no such association [Citation25]. In view of the existing literature, the results of the present study, with no association between surgeon volume or hospital volume and 5-year mortality, are not entirely unexpected. Yet, these findings are different from research examining esophageal cancer, where higher annual surgeon volume has consistently been found to be a strong and independent predictor of 5-year survival [Citation11,Citation26,Citation27]. However, there are differences in gastrectomy and esophagectomy for cancer that could explain these discrepancies. Esophagectomy includes major surgery of both the abdominal and thoracic cavity, and gastrectomy is a relatively low-risk procedure compared to esophagectomy.
The lack of association between annual surgeon and hospital volume of gastrectomies and 5-year all-cause mortality in gastric adenocarcinoma in this study might be explained by experiences gained by conducting other surgical procedures among surgeons with a lower volume of gastrectomies, resulting in surgical quality comparable with surgeons with a higher volume of gastrectomies. Another explanation may be that higher annual volumes than those captured in the present study are needed for a survival benefit to become evident. It is also possible that some more complex cases with lower chance of survival were referred to larger centers. However, the results were adjusted for age, comorbidity, and tumor stage, which should have counteracted any such influence.
The findings argue that centralization of gastric cancer surgery might not improve long-term survival. However, there are other aspects to centralization. Minimally invasive surgery is becoming increasingly common, and the associated learning curve may be reduced by higher volumes [Citation28,Citation29], and clinical research may be facilitated by centralization.
In conclusion, this population-based cohort study with adjustment for all known prognostic factors from an entire Western country with a relatively low incidence of gastric adenocarcinoma showed no long-term survival benefit of higher annual surgeon volume or hospital volume of gastrectomy for gastric adenocarcinoma.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2021;71(3):209–249.
- Ajani JA, Lee J, Sano T, et al. Gastric adenocarcinoma. Nat Rev Dis Primers. 2017;3:17036.
- Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 2019;14(1):26–38.
- Choi AH, Kim J, Chao J. Perioperative chemotherapy for resectable gastric cancer: MAGIC and beyond. World J Gastroenterol. 2015;21(24):7343–7348.
- Katai H, Ishikawa T, Akazawa K, Registration Committee of the Japanese Gastric Cancer Association, et al. Five-year survival analysis of surgically resected gastric cancer cases in Japan: a retrospective analysis of more than 100,000 patients from the nationwide registry of the Japanese Gastric Cancer Association (2001–2007). Gastric Cancer. 2018;21(1):144–154.
- Asplund J, Kauppila JH, Mattsson F, et al. Survival trends in gastric adenocarcinoma: a population-based study in Sweden. Ann Surg Oncol. 2018;25(9):2693–2702.
- Maezawa Y, Aoyama T, Kano K, et al. Impact of the age-adjusted Charlson comorbidity index on the short- and long-term outcomes of patients undergoing curative gastrectomy for gastric cancer. J Cancer. 2019;10(22):5527–5535.
- Smyth EC, Nilsson M, Grabsch HI, et al. Gastric cancer. Lancet (London, England). 2020;396(10251):635–648.
- Van Cutsem E, Sagaert X, Topal B, et al. Gastric cancer. Lancet (London, England). 2016;388(10060):2654–2664.
- Cancer AJCo. AJCC Cancer Staging Manual, Eighth Edition [Book]. 2016.
- Derogar M, Sadr-Azodi O, Johar A, et al. Hospital and surgeon volume in relation to survival after esophageal cancer surgery in a population-based study. J Clin Oncol. 2013;31(5):551–557.
- Voeten DM, Busweiler LAD, van der Werf LR, Dutch Upper Gastrointestinal Cancer Audit (DUCA) Group, et al. Outcomes of esophagogastric cancer surgery during eight years of surgical auditing by the Dutch Upper Gastrointestinal Cancer Audit (DUCA). Ann Surg. 2021;274(5):866–873.
- Iwatsuki M, Yamamoto H, Miyata H, et al. Effect of hospital and surgeon volume on postoperative outcomes after distal gastrectomy for gastric cancer based on data from 145,523 Japanese patients collected from a nationwide web-based data entry system. Gastric Cancer. 2019;22(1):190–201.
- Iwatsuki M, Yamamoto H, Miyata H, et al. Association of surgeon and hospital volume with postoperative mortality after total gastrectomy for gastric cancer: data from 71,307 Japanese patients collected from a nationwide web-based data entry system. Gastric Cancer. 2021;24(2):526–534.
- Ikoma N, Kim B, Elting LS, et al. Trends in volume–outcome relationship in gastrectomies in Texas. Ann Surg Oncol. 2019;26(9):2694–2702.
- Wu JM, Ho TW, Tien YW. Correlation between the increased hospital volume and decreased overall perioperative mortality in one universal health care system. World J Surg. 2019;43(9):2194–2202.
- Jensen LS, Nielsen H, Mortensen PB, et al. Enforcing centralization for gastric cancer in Denmark. Eur J Surg Oncol: J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2010;36(Suppl 1):S50–S54.
- Anderson O, Ni Z, Møller H, et al. Hospital volume and survival in oesophagectomy and gastrectomy for cancer. Eur J Cancer (Oxford, England: 1990). 2011;47(16):2408–2414.
- van Putten M, Nelen SD, Lemmens V, et al. Overall survival before and after centralization of gastric cancer surgery in The Netherlands. Br J Surg. 2018;105(13):1807–1815.
- Asplund J, Gottlieb-Vedi E, Leijonmarck W, et al. Prognosis after surgery for gastric adenocarcinoma in the Swedish Gastric Cancer Surgery Study (SWEGASS). Acta Oncol (Stockholm, Sweden). 2021;60(4):513–518.
- von Elm E, Altman DG, Egger M, STROBE Initiative, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–1499.
- Roderick JA, Little DBR. Statistical analysis with missing data. 3rd ed. Chichester: West Sussex: John Wiley and Sons Ltd; 2019. (Wiley Series in Probability and Statistics).
- Xirasagar S, Lien YC, Lin HC, et al. Procedure volume of gastric cancer resections versus 5-year survival. Eur J Surg Oncol: J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2008;34(1):23–29.
- Kurokawa Y, Yamaguchi T, Sasako M, et al. Institutional variation in short- and long-term outcomes after surgery for gastric or esophagogastric junction adenocarcinoma: correlative study of two randomized phase III trials (JCOG9501 and JCOG9502). Gastric Cancer. 2017;20(3):508–516.
- Mukai Y, Kurokawa Y, Takiguchi S, et al. Are treatment outcomes in gastric cancer associated with either hospital volume or surgeon volume? Ann Gastroenterol Surg. 2017;1(3):186–192.
- Lagergren J, Smyth E, Cunningham D, et al. Oesophageal cancer. Lancet (London, England). 2017;390(10110):2383–2396.
- Brusselaers N, Mattsson F, Lagergren J. Hospital and surgeon volume in relation to long-term survival after oesophagectomy: systematic review and meta-analysis. Gut. 2014;63(9):1393–1400.
- Mackenzie H, Markar SR, Askari A, et al. National proficiency-gain curves for minimally invasive gastrointestinal cancer surgery. Br J Surg. 2016;103:88–96.
- Markar SR, Ni M, Mackenzie H, et al. The effect of time between procedures upon the proficiency gain period for minimally invasive esophagectomy. Surg Endosc. 2020;34(6):2703–2708.