Background
Particularly older patients and patients with comorbidities have been reported to suffer from complications and fatalities due to coronavirus disease 2019 (COVID-19) [Citation1–5]. COVID-19 has given rise to a case-fatality rate (CFR) above 20% in cancer patients compared to 5–6% in non-cancer patients [Citation6–11]. Patients with haematological cancers have demonstrated the highest CFRs, often exceeding 30% [Citation9,Citation12,Citation13]. Emphasising the vulnerability, seroconversion 21 days after COVID-19 vaccination was 94% in healthy controls compared with 18% in patients with haematological cancer [Citation14].
Almost all previous studies have investigated prognosis using mortality rate at 30 days or CFR without specified follow-up period [Citation7,Citation15–17] and prognosis data beyond one month is scarce [Citation12,Citation18,Citation19]. Since patients with haematological cancer may have a delayed or even absent clearance of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [Citation20], studies with longer-term follow-up are relevant to monitor mortality and post infection symptoms. Recently it was reported that even SARS-CoV-2 infected patients that were not hospitalised, more frequently initiated bronchodilating agents or received a subsequent hospital diagnosis of dyspnoea than matched SARS-CoV-2 negative comparisons [Citation21]. Similarly, we found that among 66 patients with haematological disease and SARS-CoV-2 infection, 57% reported fatigue, 46% reduced functional abilities, and 33% dyspnoea one month after verified infection [Citation22]. In the present study, we extended inclusion period and follow-up to include additional patients and study mortality and functional capacity 90-days after SARS-CoV-2 infection. Herein we present data regarding clinical presentation and outcome for 108 adult Danish patients with haematological disease and SARS-CoV-2 infection verified before September 1, 2020.
Material and methods
Data sources and patients
Our study included patients with a haematological diagnosis ≥18 years with verified SARS-CoV-2 infection. Patients were in clinical follow-up at a Danish department of haematology for any subtype of haematological cancer or non-malignant blood or bone marrow disorder at the time of infection. Danish haematology departments are managed by public hospitals that provide universal free access to health care. SARS-CoV-2 testing was also facilitated by public authorities and was performed using nasal or oropharyngeal swabs and reverse transcriptase polymerase chain reaction techniques. Antigen tests was not routinely implemented during the inclusion period. Patients with respiratory tract symptoms or fever were routinely SARS-CoV-2 tested throughout the study period and by end of April 2020, extended to asymptomatic patients who were scheduled for any type of in-hospital admission. For this study, patients were identified during routine clinical practice through contacts with their haematological departments as described previously [Citation22]. The available clinical and para clinical data were abstracted from medical files by local investigators. The current study included patients for whom SARS-CoV-2 infection was verified before September 1, 2020, allowing at least three months of follow-up. Patients were eligible irrespective of severity of SARS-CoV-2 infection and therefore both hospitalised patients and patients managed at home were included. Mortality and self-reported symptoms (fatigue, dyspnoea, and functional abilities) were re-evaluated approximately three months after verified infection. Data regarding self-reported symptoms were only provided for patients evaluated through a routine virtual or an on-site hospital consultation between 80 and 120 days after confirmed infection. Follow-up terminated January 1, 2021.
Baseline data
We registered age, sex, body mass index (BMI), PS, living conditions, haematological diagnosis, previous (> 6 months), recent (0–6 months) and ongoing haematological treatment, remission status, comorbidity, and biochemical results at the time point of the verified SARS-CoV-2 infection. Charlson Comorbidity Index (CCI) and Cumulative Illness Rating Scale (CIRS) was used to summarise comorbidity [Citation23,Citation24]. Additionally, we registered comorbid conditions (e.g., diabetes and pulmonary disease) of particular interest [Citation5,Citation24]. Haematological diagnoses were aggregated in four groups including multiple myeloma (MM), chronic lymphocytic leukaemia (CLL)/lymphoma, acute leukaemia (AL)/myelodysplastic syndrome (MDS), and other haematological disorders (Chronic myeloproliferative neoplasm subtypes, chronic myeloid leukaemia, hairy cell leukaemia, T-cell large granular leukaemia, paroxysmal nocturnal haemoglobinuria, aplastic anaemia, and immune thrombocytopenia)
Definitions
SARS-CoV-2 infection was graded as either asymptomatic/mild, severe, or critical based on the available information about symptoms and observations in medical files[Citation5]. Patients with no or only mild non-pneumonia/pneumonia symptoms were classified as asymptomatic or mild. Severe infection corresponded to patients with fever, respiratory symptoms, dyspnoea, respiratory frequency ≥30/min, blood oxygen saturation ≤93%, partial pressure of arterial oxygen to fraction of inspired oxygen ratio <300, and/or radiology confirmed infiltrates in >50% of the lungs within 24 to 48 h from presentation [Citation5]. Patients with critical infection met one of three criteria: respiratory failure, septic shock, or multiple organ failure [Citation5].
Statistical analysis
We summarised baseline patient characteristics, SARS-CoV-2 findings, and outcomes using means/proportions by the pre-defined diagnosis groups and classification of SARS-CoV-2 severity. Patients were followed from the date of verified SARS-CoV-2 infection until death or censoring (January 1 2021), whichever came first. Overall survival (OS) was estimated using the Kaplan-Meier method. The risk of admission to intensive care unit (ICU) was estimated by the proportion of patients admitted to ICU within 90 days of verified infection. Ninety-five percent confidence intervals (95% CIs) for risk of ICU admission were computed according to the exact method described by Clopper and Pearson [Citation25]. Associations between SARS-CoV-2 severity or ICU admission and clinical risk factors were tested using Fishers’ exact test for grouped exposures or univariable logistic regression for continuous exposures. Analyses were conducted using the statistical programming language R (version 4.0.3).
Ethics
This study was approved by the Danish Region of Southern Denmark (record: 20/13067). The Danish council for patient safety (record: 31-1521-230) and ethics committee (record: 20202000, 53) waived informed consent due to non-interventional study design and registration of data from routine clinical care only.
Results
Baseline characteristics
The 108 included patients with haematological disorders and SARS-CoV-2 infection, had a mean age of 68.5 years (SD, 13.8) and 66 (61.1%) were male. The four aggregated diagnoses groups comprised; CLL/lymphoma (n = 49, 45.4%), MM (n = 20, 18.5%), AL/MDS (n = 14, 13.0%), and other haematological disorders (n = 25, 23.1%)) (). Therapy for the haematological disorder was ongoing in 60 (55.6%) patients, recent in 12 (11.1%), prior in 17 (15.7%) patients, and 19 (17.6%) patients were untreated (Supplementary Table 1). Prior stem cell transplantation was performed in 13 patients (3 allogeneic, 10 autologous; 12%). Comorbidity - CCI score was ≥1 in 57.4% and mean CIRS score was 6.4 (SD 4.5) (Supplementary table 1). The most common among the collected comorbid conditions were pulmonary disease (15.7%), heart disease (12.0%), and diabetes (11.1%) (Supplementary table 1). Body mass index ≥ 30 kg/m2 was present in 14.4%. Neutrophil granulocyte count was below 1.0 × 109/l in 13.8%, lymphocyte count below 1.0 × 109/l in 50.6%, and IgG blood levels below 5.0 g/l in 26.2% (Supplementary table 1).
Table 1. Clinical features and 90 day follow-up results of verfied SARS-CoV-2 infection in patients with haematological disease.
SARS-CoV-2-infection severity and treatment
Twelve (11.1%) patients were asymptomatic and among the 96 (88.8%) symptomatic patients, fever (76.0%), cough (68.8%), and dyspnoea (32.2%) were the most frequent symptoms. Asymptomatic or mild infection occurred in 57.0%, and infection was classified as severe in 31.8% and critical in 11.2% (). The proportion of mild SARS-CoV-2 infections was overall comparable across the four diagnosis groups. Hospitalisation was required in 77 (71.3%) patients and chest x-ray or CT scan was performed among 74 (68.5%) of all patients. COVID-19 pneumonia was diagnosed in 57 (52.8%), supplementary oxygen was provided to 56.5% of patients, and 21.3% required ICU transferral (). Treatment for COVID-19 was blinded as part of a clinical trial in 11 patients, open label remdesivir for 12 patients often combined with dexamethasone and <5 patients were treated with convalescent plasma, hydroxychloroquine, azithromycin, etc. The proportion of patients with severe or critical infection was higher in patients with PS ≥ 2 prior to infection (p = .05), whereas differences according to CCI, and remission status did not reach statistically significance. The proportion of patients with measurable immune deficiencies (neutropenia, lymphopenia, hypogammaglobulinaemia) was not clearly associated with the SARS-CoV-2 infection severity (Supplementary Table 2).
Overall survival
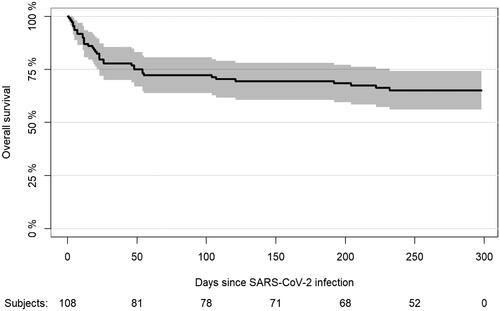
The 90-day OS following verified SARS-CoV-2 infection was 72% (95% CI, 64–81) (Supplementary Table 1, ). The 90-day OS was particularly high among patients with limited comorbidities (CCI ≤2, 78.6% [95% CI 69.8–87.3%]), ECOG PS <1 (87.2%, [95% CI, 77.7–96.8%]), age <65 years (84.8%, [95% CI, 72.6–97.1%]), and mild SARS-CoV-2 infection (90.2% [95% CI, 82.7–97.6%]). Ninety-day OS was 75.0% (95% CI, 56.0–94.0%) among patients with MM, 71.4% (95% CI, 58.8–84.1%) among patients with chronic lymphocytic leukaemia/lymphoma, 50.0% (95% CI, 23.8–76.2%) among patients with AL/MDS, and 84.0% (95% CI, 69.6–98.4%) among patients with other haematological disorders.
Among patients re-assessed after 80–120 days (n = 49), 57.8% reported to be more fatigued than usual, 54.8% reported reduced functional abilities, and 51.3% felt dyspnoea 80–120 days after verified infection (). The proportion who reported fatigue, dyspnoea, or reduced functioning was highest in patients that had experienced severe/critical infection, however differences were not statistically significant (p = .69) (Supplementary Table 2).
Discussion
In patients with haematological disorders and verified SARS-CoV-2 infection, we found that during the first three months, 21.3% were admitted to an ICU and mortality was 28%. Ninety-day OS was poorest in older patients and patients with comorbidity, but we did not have statistical power for analyses to define particularly vulnerable subgroups. For surviving patients, a considerable proportion ranging 51%-58% reported fatigue, dyspnoea, or reduced usual functioning after 90 days.
The mortality rate of 28% in our study is in line previous reports of high early mortality or case-fatality rates in haematological cancer patients following SARS-CoV-2 infection [Citation7,Citation15,Citation17,Citation19,Citation26,Citation27]. Our finding that mortality was highest in the first weeks has been observed previously in a study of 77 patients with myeloproliferative neoplasms and SARS-CoV-2 infection where OS was 55% and none of the deaths occurred after day 32 [Citation19]. Similarly, our particularly high mortality among older patients and patients with comorbidity is now a recognised characteristic of SARS-CoV-2 infection [Citation4,Citation5,Citation10,Citation27–31]. We did not observe any clear tendency towards subgroups with particular elevated mortality although mortality seemed to be highest among patients with AL/MDS. Hazard rate for death after SARS-CoV-2 infection has previously been found to be highest in AML in line with our results [Citation12].
We found that approximately 11% were asymptomatic somewhat in line with previous studies [Citation29,Citation32] and emphasising that liberal testing is warranted particularly in clinics managing vulnerable patients, such as patients with haematological disorders.
Despite the inclusion of nationwide routine data from a health care system with universal coverage and free access, our study have limitations. In the first months of the study period SARS-CoV-2 testing in Denmark was primarily performed in symptomatic patients and patients scheduled for hospitalisation. In contrast – in the last period of study inclusion large scale community testing without referral from a health care professional was initiated. Therefore it is conceivable that the capture of asymptomatic patients in the beginning of the study period was reduced. Similarly, the later widespread community testing could also reduce completeness of our study capture particular of patients with mild disease. Both these potential limitations could lead to a disproportionate inclusion of more severely affected patients with higher mortality. In addition our low number of patients prevents comprehensive statistical comparisons between clinical groups in all analyses. Of note, our mortality data are in line with most published reports.
Delayed recovery or symptoms persisting beyond four weeks after SARS-CoV-2 infection has been termed post-acute COVID-19 syndrome and may derive from complications to immunologic/inflammatory damage and known post-critical illness sequelae [Citation33]. A recent review study of this syndrome included reports with mostly 2–3 months of follow-up and found that post COVID-19 fatigue was present in 35–65% and dyspnoea in 11–43%, much like our results of persisting fatigue and dyspnoea in 58% and 51%, respectively [Citation33].
In conclusion our study confirms that mortality is high after SARS-CoV-2 infection for patients with haematological diseases. Among survivors fatigue, dyspnoea, and reduced functional capacity is very frequent even after 90 days. Since the protective effects of vaccines are considerably reduced in patients with haematological diseases optimising treatment possibly including monoclonal antibodies against SARS-CoV-2 spike protein is of paramount importance [Citation34].
Author contributions
HF conceptualised the idea for the study. H Frederiksen, A Glenthøj, L Jakobsen, J Ryg, A Kodahl, M Severinsen, and M Clausen participated in the design of the study. All authors contributed to collection of data. A Glenthøj and L Jakobsen performed data analyses and computed the figures. H Frederiksen, A Glenthøj, and L Jakobsen wrote the first draft, all authors participated in writing subsequent drafts and approved the final version.
Supplemental Material
Download MS Word (43.2 KB)Disclosure statement
The authors report no conflicts of interest.
References
- Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA. 2020;323(16):1612.
- Dai M, Liu D, Liu M, et al. Patients with cancer appear more vulnerable to SARS-COV-2: a multi-center study during the COVID-19 outbreak. Cancer Discov. 2020;10(6):783–791.
- Miyashita H, Mikami T, Chopra N, et al. Do patients with cancer have a poorer prognosis of COVID-19? An experience in New York City. Ann Oncol. 2020;31(8):1088–1089.
- Onder G, Rezza G, Brusaferro S. Case-Fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323(18):1775–1776.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72314 cases from the chinese center for disease control and prevention. JAMA. 2020;323(13):1239.
- Jensen H, Frederiksen H. Balance on slack line; diagnostic intensity and patient safety during the SARS-CoV-2 pandemic. Acta Oncol. 2021;60(1):1–3.
- Zhang H, Han H, He T, et al. Clinical characteristics and outcomes of COVID-19-Infected cancer patients: a systematic review and Meta-Analysis. J Natl Cancer Inst. 2020;113(4):371–380.
- ElGohary GM, Hashmi S, Styczynski J, et al. The risk and prognosis of COVID-19 infection in cancer patients: a systematic review and meta-analysis. Hematol Oncol Stem Cell Ther. 2020. DOI:https://doi.org/10.1016/j.hemonc.2020.07.005
- Vijenthira A, Gong IY, Fox TA, et al. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and Meta-analysis of 3377 patients. Blood. 2020;136(25):2881–2892.
- Reilev M, Kristensen KB, Pottegard A, et al. Characteristics and predictors of hospitalization and death in the first 11 122 cases with a positive RT-PCR test for SARS-CoV-2 in Denmark: a nationwide cohort. Int J Epidemiol. 2020;49(5):1468–1481.
- Ehmsen S, Jakobsen LH, Lendorf ME, et al. Severity and 1-month outcome of SARS-CoV-2 infection in patients with solid cancers: a danish nationwide cohort study. Acta Oncol. 2021;60(7):859–865.
- Passamonti F, Cattaneo C, Arcaini L, et al.; ITA-HEMA-COV Investigators. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol. 2020;7(10):e737–e745.
- Lee LYW, Cazier JB, Starkey T, et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol. 2020;21(10):1309–1316.
- Monin L, Laing AG, Munoz-Ruiz M, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22:(6)765–778.
- Cattaneo C, Daffini R, Pagani C, et al. Clinical characteristics and risk factors for mortality in hematologic patients affected by COVID-19. Cancer. 2020;126(23):5069–5076.
- Chari A, Samur MK, Martinez-Lopez J, et al. Clinical features associated with COVID-19 outcome in MM: First results from international myeloma society dataset. Blood. 2020;136(26):3033–3040.
- Hollein A, Bojko P, Schulz S, et al. Characteristics and outcomes of patients with cancer and COVID-19: results from a cohort study. Acta Oncol. 2021;60(1):24–27.
- Muntanola A, Villacampa G, Hernandez-Rivas JA, et al.; of the GELLC (Grupo Español de Leucemia Linfática Crónica). Clinical characteristics and outcome of SARS-CoV-2 infection in admitted patients with chronic lymphocytic leukemia from a single european country. Exp Hematol Oncol. 2020;9(1):37.
- Salisbury RA, Curto-Garcia N, O'Sullivan J, et al. Results of a national UK physician reported survey of COVID-19 infection in patients with a myeloproliferative neoplasm. Leukemia. 2021;35(8):2424–2430.
- Helleberg M, Niemann CU, Moestrup KS, et al. Response to aviv et al. J Infect Dis. 2021;224(3):559–561.
- Lund LC, Hallas J, Nielsen H, et al. Post-acute effects of SARS-CoV-2 infection in individuals not requiring hospital admission: a danish population-based cohort study. Lancet Infect Dis. 2021;21(10);1373–1382.
- Glenthoj A, Jakobsen LH, Sengelov H, et al. SARS-CoV-2 infection among patients with haematological disorders: Severity and one-month outcome in 66 danish patients in a nationwide cohort study. Eur J Haematol. 2020;106(1):72–81.
- Miller MD, Paradis CF, Houck PR, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the cumulative illness rating Scale. Psychiatry Res. 1992;41(3):237–248.
- Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel Coronavirus-Infected pneumonia in wuhan, China. JAMA. 2020;323(11):1061.
- Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26(4):404–413.
- Martín-Moro F, Marquet J, Piris M, et al. Survival study of hospitalised patients with concurrent COVID-19 and haematological malignancies. Br J Haematol. 2020;190(1):e16–e20.
- Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436.
- Wynants L, Van Calster B, Bonten MMJ, et al. Prediction models for diagnosis and prognosis of covid-19: systematic review and critical appraisal. BMJ. 2020;369:m1328.
- Cook G, Ashcroft AJ, Pratt G, et al.; the United Kingdom Myeloma Forum. Real-world assessment of the clinical impact of symptomatic infection with severe acute respiratory syndrome coronavirus (COVID-19 disease) in patients with multiple myeloma receiving systemic anti-cancer therapy. Br J Haematol. 2020;190(2):e83–e86.
- Kuderer NM, Choueiri TK, Shah DP, COVID-19 and Cancer Consortium, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395(10241):1907–1918.
- Karlsson LK, Jakobsen LH, Hollensberg L, et al. Clinical presentation and mortality in hospitalized patients aged 80+ years with COVID-19-a retrospective cohort study. Arch Gerontol Geriatr. 2021;94:104335.
- Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513.
- Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–615.
- Del Bello A, Marion O, Vellas C, et al. Anti-SARS-Cov-2 monoclonal antibodies in Solid-Organ-Transplant patients. Transplantation. 2021;105(10):e146–e147.