Abstract
Background
In patients with inoperable local regional recurrences of breast cancer in previously irradiated areas, local control is difficult to maintain and treatment options are limited. The Dutch standard treatment for such recurrences is reirradiation combined with hyperthermia. Apart from enhancing the effect of reirradiation, hyperthermia is also known to improve local effects of chemotherapy like cisplatin. This randomized phase-II trial compares reirradiation and hyperthermia versus the same treatment combined with cisplatin.
Patients and methods
From December 2010 up to January 2019, 49 patients were randomized, 27 in the standard arm and 22 in the combined arm. A total of 32 Gy was given in eight fractions of 4 Gy in 4 weeks, at two fractions per week. After January 2015, the radiation schedule was changed to 46 Gy in 23 fractions of 2 Gy, at five fractions per week. Hyperthermia was added once a week after radiotherapy. The combined arm was treated with four cycles of weekly cisplatin 40 mg/m2.
Results
Complete response rate was 60.9% in the standard arm and 61.1% in the combined arm (p = 0.87). Partial response rate was 30.4% in the standard arm and 33.3% in the combined arm (p = 0.79). One-year overall survival was 63.4% in the standard arm and 57.4% in the combined arm. One-year local progression-free interval was 81.5% in the standard arm and 88.1% in the combined arm (p = 0.95). Twenty-five percentage of patients in the standard arm experienced grade 3 or 4 acute toxicity and 29% of patients in the combined arm (p = 0.79).
Conclusion
No potential benefit could be detected of adding cisplatin to reirradiation and hyperthermia in patients with recurrent breast cancer in a previously irradiated area. With or without cisplatin, most patients had subsequent local control until last follow-up or death.
Background
Local control is difficult to maintain in patients with inoperable local regional recurrences of breast cancer in previously irradiated areas. Effective treatment options are limited, resulting in a high risk of uncontrollable local disease. Moreover, progression of local regional recurrences may ultimately cause ulceration with odor, pain and bleeding resulting in considerable physical and mental suffering [Citation1]. According to the Dutch National Guidelines, the standard treatment for local regional recurrent breast cancer in previously irradiated area is radiotherapy (RT), in a relatively low dose to avoid toxicity, combined with hyperthermia (HT) as a radiosensitizer [Citation2].
There is a clear clinical and preclinical rationale for using hyperthermia to improve local tumor control in patients with advanced tumors of various types at high risk of local recurrence [Citation3,Citation4]. Treatment at temperatures in the range of 40–43°C is cytotoxic for cells in an environment with a chronically low pO2 and pH. These conditions are found specifically within tumors and are associated with resistance to radiotherapy and chemotherapy [Citation5]. Hyperthermia improves tumor perfusion and oxygenation, hence sensitizing tumor cells for radiotherapy and chemotherapy. In addition, hyperthermia enhances the effect of radiotherapy by temporarily blocking DNA damage repair [Citation6–9]. Indeed, a systematic review of 38 randomized and non-randomized comparative studies showed that hyperthermia improves complete response rate of radiotherapy and/or chemotherapy in various tumor types [Citation10–16]. In a meta-analysis of 5 RT/HT phase-III trials involving 306 patients, the complete response rate of RT/HT was 59% versus 41% with RT alone [Citation17]. The greatest effect was observed in patients with recurrent lesions in previously irradiated area, and no increase of toxicity was observed. In the randomized European Society of Hyperthermic Oncology (ESHO) trial of 56 patients with recurrent breast cancer in a previously irradiated area the complete response rate was 78% in the RT/HT group versus 38% in the RT group (p = 0.004), with a three-year local control rate of 52% in the RT/HT patients.
Hyperthermia is also known to improve effects of chemotherapy both in preclinical and clinical application, particularly of platinum-based compounds like Cis-Diamino-Dichloro-Platinum (cDDP) or cisplatin [Citation16,Citation18–21]. Thermal enhancement ratio (TER) is defined as the percentage of increased cell kill that is achieved by adding hyperthermia to radiotherapy or chemotherapy. Hyperthermia added to cisplatin shows an excellent TER [Citation19,Citation20]. Radiotherapy combined with cisplatin is a well-known chemoradiotherapy combination, with mutual effect enhancement, for instance in lung cancer [Citation22] and uterine cervix cancer [Citation23]. In the latter, the combination of radiotherapy, hyperthermia and cisplatin 40 mg/m2 appears feasible and effective [Citation24–26]. Cisplatin is generally not used in the primary treatment of breast cancer, nor in the treatment of recurrent breast cancer. Most patients with a recurrence of breast cancer have been treated with anthracyclines and/or taxanes as part of their primary breast cancer treatment, either in neo-adjuvant or in adjuvant setting according to the Dutch National Guidelines [Citation2]. There is no cross-resistance between cisplatin and anthracyclines or taxanes, subsequently making cisplatin resistance less likely [Citation14–18]. The triple combination of radiotherapy, cisplatin and hyperthermia has also been used in the treatment of breast cancer [Citation27–29]. For breast cancer, this combination has previously demonstrated a complete response rate of 53% [Citation28]. In view of the established radio-enhancing effect of cisplatin and the enhancement of cisplatin and radiotherapy by hyperthermia, one might hypothesize that this triple-modality treatment can lead to improvement of local control, although toxicity may also be enhanced. Therefore, this study performed a randomized phase-II trial exploring the feasibility and efficacy of RT/HT versus RT/HT with cisplatin. The aim of this study was to assess feasibility and potential efficacy by comparing local progression-free interval, survival and toxicity with RT/HT with or without cisplatin in patients with a macroscopic locoregional recurrence of breast cancer in a previously irradiated area.
Patients and methods
Patients
Patients with macroscopic locoregional recurrence of breast cancer in previously irradiated area not suitable for resection were enrolled from December 2010 up to January 2019. To be eligible, the locoregional recurrence had to be measurable by clinical examination and/or radiological assessment, and confirmed by histology or fine needle aspiration (FNA). Furthermore, Eastern Cooperative Oncology Group (ECOG) performance score had to be 0–2. Distant metastases were allowed if life expectancy was ≥1 year. Concurrent hormonal therapy was allowed. Patients required adequate bone marrow, hepatic and renal function. Patients were excluded if they had uncontrolled infection, other previous malignancies or a pacemaker or implanted defibrillator on the same site as the treatment area.
Patients were randomized to treatment with RT/HT (standard arm) or RT/HT with cisplatin (combined arm). The random permuted blocks randomization was employed. Randomization took place at the Academic Medical Center (AMC) and was stratified by size of recurrence (>5cm or ≤ 5 cm) and time interval between primary breast cancer and first recurrence (>3 y or ≤3 y).
Sample size
The study was designed with 90% power to detect an increase in the local control rate after 1 year from 54% in the standard treatment arm to 69% in the study arm (corresponding to a hazard ratio of 0.6) at a 20% level of statistical significance. This required 71 local relapses to be observed. This could be achieved by recruiting 90 patients over 4.5 years (20 patients/year) and following these patients for an additional 1.5 years after completion of accrual. We assumed a dropout rate of 10% due to patient withdrawal, lost to follow up or death. Therefore, a total of 104 patients (52 patients per arm) was required, lengthening the accrual time to 5.2 years.
Treatment
Radiotherapy
A total of 32 Gy was given in eight fractions of 4 Gy in 4 weeks, at two fractions per week (3 days in between the fractions). After January 2015, the radiotherapy schedule was changed to 46 Gy in 23 fractions of 2 Gy, at five fractions per week. The 2 Gy fraction schedule was expected to have less late side effects and to result in a better connection with (inter)national institutes. For the 8 × 4 schedule, the EQD2 with an α/β ratio of 3 is 44.8 Gy, being nearly 23 × 2 Gy. The α/β of 3 was used for breast cancer as well as for late responding normal tissue (thus late toxicity of normal tissue). It was assumed that breast cancer responds in a similar manner as late responding normal tissue, reflecting the slower proliferation rate of breast cancer (i.e., compared with high α/β ratio of over 8 for squamous cell carcinomas). Megavoltage equipment was used with photon energies of 6 or 10 MV and electron energies of 6–15 MeV. Until 2012 a technique of combined photon and electron fields was used as described earlier [Citation30]. After 2012, intensity-modulated radiotherapy (IMRT) became the standard technique and since 2016 volumetric modulated arc radiotherapy (VMAT) planning was used.
Hyperthermia
Local microwave (MW) hyperthermia was delivered once a week, starting within 1 h after radiotherapy. Patients receiving the 8 × 4 Gy schedule were given four sessions of hyperthermia and patients receiving the 23 × 2 Gy schedule were given five sessions of hyperthermia. For superficial hyperthermia (reaching up to 40 mm beneath the skin surface), a Contact Flexible Microstrip Antenna with water bolus operating at a frequency of 434 MHz was used (ISTOK, Fryazino, Russia; after 2014 Medlogix, Rome, Italy). Five different antenna sizes were available. The antenna was chosen according to its effective field size and the surface of the target area. Up to eight multi-sensor temperature probes (Ella CS, Czech Republic) were placed onto the skin in and near the tumor target region. One or two small catheters containing multi-sensor temperature probes were inserted into the macroscopic tumor under local anesthesia at the discretion of the treating physician. The goal for each hyperthermia treatment was to achieve a minimal intratumoral temperature exceeding 40 °C and a median intratumoral temperature exceeding 41 °C. This condition had to be maintained for at least 60 min in accordance with ESHO Quality Assurance (QA) Guidelines [Citation31]. For adjacent normal tissue the maximum temperature was limited at 42 °C. The AMC-2 Phased Array hyperthermia system with waveguides operating at 70 MHz was used if the recurrence was located deeper than 40 mm from skin surface, for instance in case of an axillary lymph node recurrence [Citation32]. In these cases, the same temperature constraints were maintained as for superficial hyperthermia.
Cisplatin
Patients were treated with weekly cisplatin 40 mg/m2 given intravenously for four courses with appropriate antiemetic premedication and hydration according to institutional protocol. The patients receiving five hyperthermia sessions received one session without cisplatin. Cisplatin was given concurrently with hyperthermia, thus starting within 1 h after irradiation. Cisplatin was given in 1½ h in 250 ml NaCl 3% after urinary output was >100 cc/hr. If cisplatin-related toxicities occurrences could not be managed with standard supportive care, cisplatin dose was reduced in steps of 25%.
Endpoints and data analysis
Treatment response
Local response was measured according to the RECIST (Response Evaluation Criteria In Solid Tumors) criteria [Citation33]. Evaluation of tumor response was determined clinically by the treating physician or radiologically if the tumor could not be measured clinically. The best response achieved by a patient during follow-up was used regardless of when this response occurred. Patients who withdrew participation before finishing study treatment were considered to be non-responders, as well as patients who were removed due to lost to follow up or patients who died before any response assessment.
Local (infield) progression-free interval (LPFI)
Local (infield) progression-free interval (LPFI) was calculated from the date of randomization until infield progression occurred. Infield progression was defined as local progression within the irradiated area after initial complete response (CR), partial response (PR) or stable disease (SD). When progressive disease (PD) occurred, time to disease failure was set from the date of randomization. Patients without local progression were censored at the date of last follow-up. Patients who died without local progression were censored at date of death.
Overall survival
Overall survival (OS) was calculated from the date of randomization until the date of death of any cause. Patients who withdrew participation early in the study were censored at the date of last follow-up or the date of withdrawal.
Toxicity
Toxicity was measured by the treating physician according to the Common Terminology Criteria of Adverse Events (CTCAE v4.0). As a safety rule, treatment was stopped if dermatitis grade 4 occurred. This was defined as skin necrosis and/or ulceration induced by treatment, requiring surgery and/or hyperbaric oxygen. In case of renal function disturbances, cisplatin dose was reduced in steps of 25%. If recovery did not occur, chemotherapy was stopped. Any other unanticipated grade 4 side effect according to the CTCAE led to discontinuation of study treatment. Furthermore, if the tumor was ulcerative before treatment and the ulceration persisted after treatment, this was not assessed as acute toxicity. Toxicity was assessed during treatment and at every follow-up visit until 3 months after treatment.
Follow-up
Patients were seen 2, 4 and 6 weeks and 3, 6, 9, and 12 months after completion of protocol therapy. Thereafter patients were seen every 6 months or earlier in case of complaints or suspicion of recurrence. If CR or PR was assessed at one of these visits, the patient was seen 4 weeks after that visit for confirmation according to the RECIST criteria [Citation33].
Statistics
Statistics were performed using SPSS version 26 and R version 3.6.3. All analyses were conducted on an intention to treat basis. Comparisons between groups were analyzed by one-way ANOVA. LPFI and OS were presented graphically using Kaplan–Meier curves. Log-rank test was used to compare outcomes of the groups. The influence of various factors on LPFI and OS (age, location of the local relapse, the number of relapses, the type of relapse, presence of distant metastases (DM), the presence of regional relapse, the presence of contralateral relapse, salvage mastectomy and treatment started before the study treatment: hormonal therapy, chemotherapy or therapy with Trastuzumab) were investigated using univariate and multivariate (backward stepwise) Cox Proportional-Hazards Model. The results were graphically presented using forest plots. The median survival time was calculated using the inverse Kaplan–Meier method. Significance was set at p ≤ 0.05.
Results
Patient characteristics
Between April 2010 and January 2019, 49 patients were randomized, 27 in the standard arm and 22 in the combined arm. Initial accrual was eight patients/year but since 2015 many patients were treated with chemotherapy followed by a resection of the relapse before referral to our department. Hence accrual rate dropped to three patients per year. Therefore, in April 2021, the study was closed. Patient characteristics are listed in . Baseline characteristics did not vary significantly between the arms. Median age was 60 in the standard arm and 58 in the combined arm (p = 0.72). The standard arm contained more patients with distant metastases (57% vs. 38%), but this was not statistically significant (p = 0.19). Most patients had primarily been operated (93% vs. 100%, p = 0.22) in combination with chemotherapy (71% vs. 76%, p = 0.72). The type of relapse treated in this study was mostly lymphangitis cutis (36% vs. 62%, p = 0.31) and most patients had additional involvement of regional nodes (71% vs. 71%, p = 0.84). Approximately half of the patients had already been unsuccessfully treated for the current relapse with surgery, chemotherapy, hormonal therapy or targeted therapy (i.e., trastuzumab) (43% vs. 52%, p = 0.88).
Table 1. Patient characteristics.
Treatment compliance
In the standard arm, 24 out of 27 patients started treatment and in the combined arm 20 out of 22 patients started treatment. Two patients in the standard arm and one patient in the combined arm were unable to start treatment due to new metastases on PET-CT. One patient in the standard arm was excluded after it became apparent that the local recurrence was not located in a previously irradiated area. One protocol violation occurred when a patient randomized in the combined treatment was wrongly treated in the standard arm from the start. This patient has been analyzed as part of the standard arm. In the standard arm, 23 out of 24 patients completed treatment, and in the combined arm, 18 out of 20 patients completed treatment. One patient in each arm discontinued treatment due to new metastases emerging during therapy. One patient in the combined arm died of metastatic breast cancer prior to response assessment after finishing treatment. In the standard treatment arm, one patient only received three sessions of hyperthermia due to skin burn grade 3. In the combined arm, 52% of the starting patients finished chemotherapy according to plan. Three patients only received two courses of chemotherapy and six patients received three courses due to toxicity. Two patients continued chemotherapy successfully with a 25% dose reduction. One patient started chemotherapy with daily 6 mg/m2 dosage due to impaired renal function and finished treatment in this regimen.
Clinical outcome
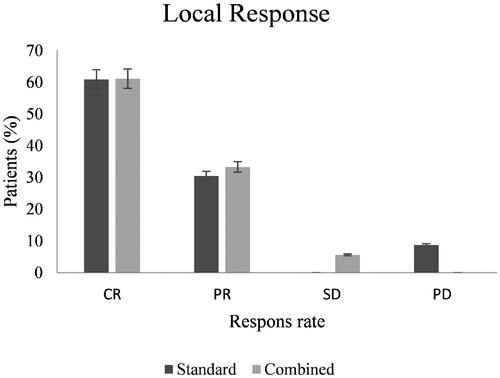
CR was 60.9% in the standard treatment arm and 61.1% in the combined treatment arm (p = 0.87) (). PR was 30.4% in the standard treatment arm and 33.3% in the combined treatment arm (p = 0.79). In the combined treatment arm, 5.6% had SD and none in the standard arm (p = 0.20). In the standard treatment arm, 8.7% had PD and none in the combined arm (p = 0.22).
Figure 1. Treatment response. Bar graph of assessed local response in both study arms. CR: complete response; PR: partial response; SD: stable disease; PD: progressive disease.
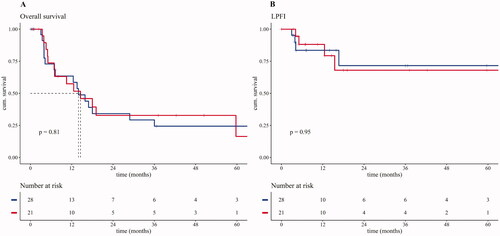
The median OS was 13.9 months in the standard arm and 14.5 months in the combination arm (p = 0.81). One-year OS was 63.4% in the standard arm and 57.4% in the combined arm (). One-year LPFI was 81.5% in the standard arm and 88.1% in the combined arm (p = 0.95) with a median follow-up time of 7.14 months in the standard arm and 12.6 months in the combined arm (p = 0.39).
Figure 2. Kaplan–Meijer curves. (A) Kaplan–Meijer curve comparing the overall survival of both study groups. Median survival of both groups depicted in dotted lines. Beneath the Kaplan–Meijer curve the number at risk is described as the number still alive over time in months. (B) Kaplan–Meijer curve comparing the LPFI of both study groups. Beneath the Kaplan–Meijer curve the number at risk is described as the number without local progression over time in months.
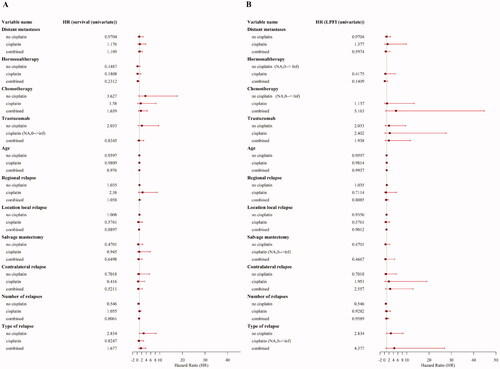
A total of 11 treatment related variables were included in an univariate analysis of LPFI and OS. None of these variables were associated with a significant benefit on overall survival (), except for concurrent hormonal therapy which showed a significant association with LPFI.
Figure 3. Forest plots of univariate analyses. (A) Forest plot presenting the estimated effect of different variables on overall survival (OS) using the hazard ratio. Variables are shown describing the effect in the cisplatin and the noncisplatin group as well as the combined effect on OS. (B) Forest plot presenting the estimated effect of different variables on local progression-free interval (LPFI) using the hazard ratio. Variables are shown describing the effect in the cisplatin and the non-cisplatin group as well as the combined effect on LPFI.
Toxicity
Toxicity outcomes per study arm are listed in . Twenty-five percentage of patients in the standard arm experienced grade 3 or 4 acute toxicity and 29% of patients in the combined arm (p = 0.79). Most frequent graded toxicity was radiodermatitis, which occurred in 12 patients. Hyperthermia had caused skin burn grade 3 in one case of the standard arm. Four patients in the combined arm had to stop chemotherapy because of toxicity grade 4, mostly due to temperature probe related infection. One patient in the standard arm died due to a necrotizing thoracic wall defect in the radiated area 3 months after treatment.
Table 2. Number of patients with high-graded toxicity.
Discussion
This randomized, open label phase-2 study, aimed to assess the feasibility and potential efficacy of adding cisplatin to reirradiation and hyperthermia for locoregional breast cancer recurrence, found no significant difference in outcome. Only limited data are available on the efficacy of combined radiotherapy, hyperthermia and chemotherapy in recurrent breast cancer. This is the first randomized trial comparing dual- and triple-modality therapy in patients with recurrence of breast cancer in previously irradiated area. However, due to lack of accrual, the target sample size could not be reached and analysis was underpowered.
Patients with local recurrent breast cancer in previously irradiated area who are candidates for reirradiation with hyperthermia, inherently have a relatively poor prognosis with a median survival rate of little more than one year. Importantly, in both study arms, the majority of patients did not suffer subsequent local progression during the rest of their life before passing away. This might indicate that the addition of cisplatin to the standard treatment of radiotherapy and hyperthermia did not show an additional effect on tumor control. Median follow-up time of patients with LPFI was short mainly due to decreased OS in this patient cohort.
This study’s clinical outcomes are in line with those of other studies focusing on triple-modality therapy in recurrent breast cancer. In a similar study, CR was achieved in 53% with a median duration of 7 months [Citation28]. Other studies used doxorubicin encapsulated in liposomes, facilitating the infiltration of tumor tissue and preventing severe systemic toxicity [Citation34]. With an overall local response rate of 48% and a median time to local progression of 4.9 months [Citation35], this type of chemotherapy does not seem to surpass this study’s results with or without cisplatin. The chemotherapy regimen of capecitabine, vinorelbine and paclitaxel showed better response rates (80% CR and one-year LPFI was 76%) compared to chemotherapy regimen used in this study [Citation29]. However, not all patients included in the Zagar et al. study was reirradiated and patients lost to follow up were censored from analysis.
Some toxicity reports had to be graded retrospectively based on the description of the physician. This could potentially have led to under-reporting of toxicity. Nonetheless, high rates of toxicity were reported in this study. These high toxicity rates are in accordance with earlier studies and can be explained by the fact that all recurrences were located in previously operated and irradiated areas [Citation36]. Hence, the late toxicity may be considered as the sum of the toxicity from previous and current treatments. Due to heterogeneity, for example in terms of study population, comparing toxicity scores is challenging. Other studies using the same 8 × 4 Gy schedule for irresectable recurrent breast cancer in previously irradiated area reported acute toxicity based on CTC-criteria. These studies showed acute high-grade toxicity in 24–33% of patients [Citation30,Citation37]. These findings are in line with the results of this study. Significant differences in acute toxicity rates comparing both study-arms could not be found, suggesting the addition of cisplatin does not increase severe acute toxicity. After January 2015, the radiotherapy schedule was changed to 46 Gy in 23 fractions of 2 Gy, at five fractions per week. The 2 Gy fraction schedule was expected to have less late side-effects. However, this study did not report toxicity after 3 months so late side-effects could not be evaluated.
The tumor depth in this study did not exceed the maximum heating depth achievable for the used hyperthermia devices and achieving therapeutic tumor heating at depth exceeding 1 cm from the skin was always verified with invasive thermometry probes according to protocol. Our target temperature of 41–43°C is conform to ESHO QA Guidelines [Citation31] and provides proven synergy with radiotherapy [Citation17]. The mechanisms of hyperthermia suggest a dose–effect relationship, for instance for the inhibition of homologous recombination [Citation38]. Bergs et al. [Citation39] concluded that heating to 41 °C already yields excellent synergistic interaction in cisplatin-sensitive cell lines. However, in cisplatin-resistant cell lines temperature had to be increased to 43 °C to gain the same therapeutic effect. This temperature is less easily achieved in the entire tumor target and causing more risk of skin burns, as shown in Bakker et al [Citation40]. Cisplatin is not used for the treatment of primary breast cancer, making resistance less likely [Citation2]. Nevertheless, a tumor can still consist of multiple malignant clones with variable degrees of cisplatin sensitivity, making it difficult to achieve optimal therapeutic temperature in clinical practice. Hyperthermia at temperatures of 41 °C already shows clinical results in patients with recurrent breast cancer [Citation41]. Thus, operators always aim for maximum achievable tumor temperatures within the 41–43 °C range, but avoid skin temperatures exceeding 43 °C.
Also, timing of the three modalities is important. Simultaneous administration of all three components may be expected to give optimal synergy, but this is challenging for technical reason. We gave radiotherapy first, followed 1 h later by simultaneous cisplatin and hyperthermia. This short time interval between radiotherapy and hyperthermia is expected to combine optimal radiosensitization with a limited risk of adding toxicity [Citation42]. However, data on optimal treatment sequence in combined radiotherapy, hyperthermia and chemotherapy is scarce. Preclinical and clinical results suggest that the benefit of adding hyperthermia before or after radiotherapy is similar [Citation42,Citation43]. Triple combination of cisplatin, radiotherapy and hyperthermia was successfully applied in randomized trials with similar timing schedules for other tumor sites including locally advanced cervical cancer [Citation25,Citation26,Citation44].
Hyperthermia is known to temporarily improve tumor perfusion and thereby reduce hypoxia and treatment-resistance [Citation6,Citation45]. Also, hyperthermia indirectly reduces tumor hypoxia by the selective killing of hypoxic cells [Citation46]. A direct synergistic mechanism of hyperthermia is the temporary inhibition of DNA damage repair induced by cisplatin and radiotherapy. This mechanism can be observed when temperatures exceed 41 degrees [Citation47]. One of the targeted repair pathways includes the degradation of the essential breast cancer susceptibility gen 2 (BRCA2), thereby inhibiting homologous recombination [Citation7]. This provides an opportunity for novel treatment strategies by combine hyperthermia with other targeted therapies [Citation48]. For example, PARP-inhibitors like Olaparib are presently used on a small scale in breast cancer for patients with a BRCA-mutation. Hyperthermia has the potential to induce a BRCA2 depletion in the heated tumor, thus expanding the group of patients who can benefit from Olaparib treatment [Citation49]. In a study of Vriend et al., HSP90-inhibitor Ganetespib was used in combination with hyperthermia in cervix cancer cell lines [Citation50]. Addition of these inhibitors enhanced BRCA2 degradation, making it possible to achieve the same therapeutic effect in a shorter hyperthermia treatment. Thus, patients with resistant or hypoxic recurrences may benefit from hyperthermia treatment combined with systemic targeted therapies, such as Olaparib.
Conclusion
In this prematurely closed, randomized phase-2 trial no potential benefit could be detected of adding cisplatin chemotherapy to reirradiation and hyperthermia in patients with recurrent breast cancer in a previously irradiated area. With or without cisplatin, most patients had subsequent local control until last follow-up or death.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Bedwinek JM, Fineberg B, Lee J, et al. Analysis of failures following local treatment of isolated local-regional recurrence of breast cancer. Int J Radiat Oncol Biol Phys. 1981;7(5):581–585.
- Richtlijn Borstkanker [Internet]. 2017. [cited 2021 Apr 18]. Available from: http://www.vkng.org.
- van der Zee J. Heating the patient: a promising approach? Ann Oncol. 2002;13(8):1173–1184.
- Falk MH, Issels RD. Hyperthermia in oncology. Int J Hyperthermia. 2001;17(1):1–18.
- Overgaard J. Hypoxic modification of radiotherapy in squamous cell carcinoma of the head and neck–a systematic review and meta-analysis. Radiother. Oncol. 2011;100(1):22–32.
- Dewhirst MW, Vujaskovic Z, Jones E, et al. Re-setting the biologic rationale for thermal therapy. Int J Hyperthermia. 2005;21(8):779–790.
- Krawczyk PM, Eppink B, Essers J, et al. Mild hyperthermia inhibits homologous recombination, induces BRCA2 degradation, and sensitizes cancer cells to poly (ADP-ribose) polymerase-1 inhibition. Proc Natl Acad Sci USA. 2011;108(24):9851–9856.
- Elming PB, Sorensen BS, Oei AL, et al. Hyperthermia: the optimal treatment to overcome radiation resistant hypoxia. Cancers. 2019;11(1):60.
- Oei AL, Kok HP, Oei SB, et al. Molecular and biological rationale of hyperthermia as radio- and chemosensitizer. Adv Drug Deliv Rev. 2020;163–164:84–97.
- Datta NR, Ordonez SG, Gaipl US, et al. Local hyperthermia combined with radiotherapy and-/or chemotherapy: recent advances and promises for the future. Cancer Treat Rev. 2015;41(9):742–753.
- van der Zee J, van der Holt B, Rietveld PJM, et al. Reirradiation combined with hyperthermia in recurrent breast cancer results in a worthwhile local palliation. Br J Cancer. 1999;79(3–4):483–490.
- Jones EL, Oleson JR, Prosnitz LR, et al. Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol. 2005;23(13):3079–3085.
- Jones EL, Marks LB, Prosnitz LR. Point: hyperthermia with radiation for chest wall recurrences. J Natl Compr Canc Netw. 2007;5(3):339–344.
- Li G, Mitsumori M, Ogura M, et al. Local hyperthermia combined with external irradiation for regional recurrent breast carcinoma. Int J Clin Oncol. 2004;9(3):179–183.
- Wahl AO, Rademaker A, Kiel KD, et al. Multi-Institutional review of repeat irradiation of chest wall and breast for recurrent breast cancer. Int J Radiat Oncol Biol Phys. 2008;70(2):477–484.
- Overgaard J, Radacic MM, Grau C. Interaction of hyperthermia and m-Diamminedichloroplatinum(II) alone or combined with radiation in a C3H mammary carcinoma in vivo. Cancer Res. 1991;51(2):707–711.
- Vernon CC, Hand JW, Field SB, et al. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: results from five randomized controlled trials. Int J Radiat Oncol Biol Phys. 1996;35:731–744.
- van Driel WJ, Koole SN, Sikorska K, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med. 2018;378(3):230–240.
- Rietbroek RC, Schilthuis MS, Bakker PJM, et al. Phase II trial of weekly locoregional hyperthermia and cisplatin in patients with a previously irradiated recurrent carcinoma of the uterine cervix. Cancer. 1997;79(5):935–943.
- Hettinga JVE, Konings AWT, Kampinga HH. Reduction of cellular cisplatin resistance by hyperthermia-a review. Int J Hyperthermia. 1997;13(5):439–457.
- Rietbroek RC, Schilthuis MS, van der Zee J, et al. Hyperthermia in combination with chemotherapy in gynaecological tumours. Ned Tijdschr Geneeskd. 1999;143:85–88.
- Schaake-Koning C, van den Bogaert W, Dalesio O, et al. Effects of concomitant cisplatin and radiotherapy on inoperable non-small-cell lung cancer. N Engl J Med. 1992;326(8):524–530.
- Pearcey R, Brundage M, Drouin P, et al. Phase III trial comparing radical radiotherapy with and without cisplatin chemotherapy in patients with advanced squamous cell cancer of the cervix. J Clin Oncol. 2002;20(4):966–972.
- Westermann AM, Jones EL, Schem BC, et al. First results of triple-modality treatment combining radiotherapy, chemotherapy, and hyperthermia for the treatment of patients with stage IIB, III, and IVA cervical carcinoma. Cancer. 2005;104(4):763–770.
- Ohguri T, Harima Y, Imada H, et al. Relationships between thermal dose parameters and the efficacy of definitive chemoradiotherapy plus regional hyperthermia in the treatment of locally advanced cervical cancer: data from a multicentre randomised clinical trial. Int J Hypertherm. 2018;34(4):461–468.
- Wang Y, Hong W, Che S, et al. Outcomes for hyperthermia combined with concurrent radiochemotherapy for patients with cervical cancer. Int J Radiat Oncol Biol Phys. 2020;107(3):499–511.
- Herman TS, Teicher BA. Summary of studies adding systemic chemotherapy to local hyperthermia and radiation. Int. J. Hypertherm. 1994;10(3):443–449.
- Bornstein BA, Zouranjian PS, Hansen JL, et al. Local hyperthermia, radiation therapy, and chemotherapy in patients with local-regional recurrence of breast carcinoma. Int J Radiat Oncol Biol Phys. 1993;25(1):79–85.
- Zagar TM, Higgins KA, Miles EF, et al. Durable palliation of breast cancer chest wall recurrence with radiation therapy, hyperthermia, and chemotherapy. Radiother Oncol. 2010;97(3):535–540.
- Oldenborg S, Griesdoorn V, van Os R, et al. Reirradiation and hyperthermia for irresectable locoregional recurrent breast cancer in previously irradiated area: size matters. Radiother Oncol. 2015;117(2):223–228.
- Trefna HD, Crezee H, Schmidt M, et al. Quality assurance guidelines for superficial hyperthermia clinical trials: I. Clinical requirements. Int J Hypertherm. 2017;33(4):471–482.
- van Stam G, Kok HP, Hulshof MCCM, et al. A flexible 70 MHz phase-controlled double waveguide system for hyperthermia treatment of superficial tumours with deep infiltration. Int J Hyperthermia. 2017;33(7):796–809.
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216.
- Kouloulias VE, Dardoufas CE, Kouvaris JR, et al. Liposomal doxorubicin in conjunction with reirradiation and local hyperthermia treatment in recurrent breast cancer: a phase I/II trial. Clin Cancer Res. 2002;8:374–382.
- Zagar TM, Vujaskovic Z, Formenti S, et al. Two phase I dose-escalation/pharmacokinetics studies of low temperature liposomal doxorubicin (LTLD) and mild local hyperthermia in heavily pretreated patients with local regionally recurrent breast cancer. Int J Hyperth. 2014;30(5):285–294.
- Lindholm CE, Kjellen E, Nilsson P, et al. Microwave-induced hyperthermia and radiotherapy in human superficial tumours: clinical results with a comparative study of combined treatment versus radiotherapy alone. Int J Hyperth. 1987;3(5):393–411.
- Oldenborg S, Rasch CRN, van Os R, et al. Reirradiation + hyperthermia for recurrent breast cancer en cuirasse. Strahlenther Onkol. 2018;194(3):206–214.
- van den Tempel N, Laffeber C, Odijk H, et al. The effect of thermal dose on hyperthermia-mediated inhibition of DNA repair through homologous recombination. Oncotarget. 2017;8(27):44593–44604.
- Bergs JWJ, Haveman J, ten Cate R, et al. Effect of 41 °C and 43 °C on cisplatin radiosensitization in two human carcinoma cell lines with different sensitivities for cisplatin. Oncol Rep. 2007;18:219–226.
- Bakker A, Kolff MW, Holman R, et al. Thermal skin damage during reirradiation and hyperthermia is time-temperature dependent. Int J Radiat Oncol Biol Phys. 2017;98(2):392–399.
- Bakker A, van der Zee J, van Tienhoven G, et al. Temperature and thermal dose during radiotherapy and hyperthermia for recurrent breast cancer are related to clinical outcome and thermal toxicity: a systematic review. Int J Hyperth. 2019;36(1):1023–1038.
- Overgaard J. Simultaneous and sequential hyperthermia and radiation treatment of an experimental tumor and its surrounding normal tissue in vivo. Int J Radiat Oncol Biol Phys. 1980;6(11):1507–1517.
- Datta NR, Puric E, Klingbiel D, et al. Hyperthermia and radiation therapy in locoregional recurrent breast cancers: a systematic review and meta-analysis. Int J Radiat Oncol Biol Phys. 2016;94(5):1073–1087.
- Flameling B, Nordberg T, Ott O, et al. An international multicenter phase III study of chemoradiotherapy versus chemoradiotherapy plus hyperthermia for locally advanced cervical cancer. J Clin Oncol. 2016;34(15_suppl):e17023.
- Song CW. Effect of local hyperthermia on blood flow and microenvironment: a review. Cancer Res. 1984;44:4721–4730.
- Crezee H, van Leeuwen CM, Oei AL, et al. Thermoradiotherapy planning: integration in routine clinical practice. Int J Hyperth. 2016;32(1):41–49.
- Oei AL, Vriend LEM, Crezee J, et al. Effects of hyperthermia on DNA repair pathways: one treatment to inhibit them all. Radiation Oncol. 2015;10:165.
- Issels R, Kampmann E, Kanaar R, et al. Hallmarks of hyperthermia in driving the future of clinical hyperthermia as targeted therapy: translation into clinical application. Int J Hyperth. 2016;32(1):89–95.
- van den Tempel N, Odijk H, van Holthe N, et al. Heat-induced BRCA2 degradation in human tumours provides rationale for hyperthermia-PARP-inhibitor combination therapies. Int J Hyperth. 2018;34(4):407–414.
- Vriend LEM, van den Tempel N, Oei AL, et al. Boosting the effects of hyperthermia-based anticancer treatments by HSP90 inhibition. Oncotar. 2017;8(57):97490–97503.
