 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
This article aims to evaluate the impact of smoking status, accumulated tobacco exposure (ATE), and smoking cessation on overall- and disease-free survival (OS and DFS) of patients with oral squamous cell carcinoma (OSCC).
Material and Methods
Patients with primary OSCC treated with curative intent between 2000 and 2019 in Copenhagen were included (n = 1808). Kaplan–Meier curves and multivariable Cox regression analyses were performed to compare the survival of patients with different smoking history. Interactions between ATE and (A) tumor subsite and (B) excessive alcohol consumption (EAC) on the survival were evaluated using multivariable Cox regression analyses with interaction terms.
Results
We included 1717 patients with known smoking status (62.8% males, median age: 64 years (IQR: 57–71 years)), who had a 5-year OS of 53.7% (95%CI: 49.8%–57.9%). Based on fully adjusted multivariable Cox regression analyses, significantly elevated hazard ratios (HRs) for OS and DFS were identified for current, but not former smokers, compared to never-smokers. An approximately linear relationship between continuous ATE and survival estimates was identified. ATE analyzed as a categorical variable showed significantly elevated HRs for OS of patients with all categories (0x
30, 30
x
60, and
60 PYs), however only for DFS of patients with >60 PYs, compared to 0 PYs. Furthermore, an unfavorable long-term prognosis was evident after >3.5 (OS) and >2.5 (DFS) years from diagnosis for patients who continued smoking compared to patients with smoking cessation at diagnosis. The survival estimates of patients with different tumor subsite and alcohol consumption differed with increasing ATE.
Conclusion
Tobacco smoking (assessed as smoking status and ATE) was associated with inferior survival (OS and DFS) among patients with OSCC. Unfavorable long-term prognosis was significant for patients who continued smoking compared to patients with smoking cessation at diagnosis. The impact of ATE on survival of patients with OSCC may depend on the tumor subsite and/or alcohol consumption.
Background
Oral squamous cell carcinoma (OSCC), a subgroup of head and neck squamous cell carcinoma (HNSCC), accounts for more than 90% of malignancies affecting the oral cavity [Citation1]. The worldwide incidence of OSCC is 378,000 cases a year [Citation2] and has been increasing in several countries [Citation3–5]. Tobacco smoking is a well-established risk factor for OSCC [Citation6–9] and is known to have a synergistic effect with alcohol [Citation10–12]. The 5-year overall survival (OS) for oral cancer has been estimated to 45%, with an improving tendency in many European countries [Citation5,Citation13] and the United States [Citation14]. Several studies have reported that smokers have an inferior survival in comparison with never-smokers in HNSCC [Citation15–18]. Previous studies [Citation19–29] on the impact of smoking on the survival of patients with OSCC have had conflicting results. Some articles conclude that smoking impacts OS, recurrence-free survival (RFS), and disease-specific survival (DSS) significantly negatively [Citation20,Citation23,Citation24], while others identify a significantly protective effect of smoking [Citation25] or no significant differences in survival when stratifying for smoking status [Citation19,Citation26,Citation28,Citation29].
The effect of altering smoking habits at the time of diagnosis is clinically interesting, and a previous study on HNSCC [Citation30] showed a significant effect of smoking cessation at diagnosis on the survival estimates. Smoking cessation after diagnosis may have a direct effect on postoperative wound healing [Citation31,Citation32], response to radiotherapy [Citation33], and more long-term effects such as increasing the risk of second primary tumors [Citation34,Citation35] and smoking-associated co-morbidities, thereby improving survival. The U.S. Department of Health and Human Services reviewed this topic and concluded that the evidence was suggestive but not sufficient to infer a causal relationship between smoking cessation and improved survival of patients who are smokers at the time of a cancer diagnosis [Citation36].
A significant impact of accumulated tobacco exposure (ATE, i.e. number of packyears (PYs)) prior to diagnosis on survival has been identified for several cancer types [Citation37–39]. However, only one study on OSCC [Citation22] has previously analyzed the effect of the ATE history in a dose-dependent fashion.
Conflicting results and scarce data on ATE emphasized the need for a larger cohort study that evaluates the effect of smoking on survival of patients with OSCC while integrating both categorical and continuous quantifications of smoking history and adjusting for the numerous other factors affecting the survival. This article aims to evaluate the impact of smoking status, ATE, and smoking cessation on overall- and disease-free survival (OS and DFS) of patients with OSCC.
Material and methods
Patients with primary OSCC treated with curative intent (following the Danish national guidelines for treatment of OSCC [Citation40]) were included from the Copenhagen Oral Cavity Squamous Cell Carcinoma (COrCa) database [Citation41]. This cohort consists of cases diagnosed and/or treated at the University Hospital of Copenhagen, Rigshospitalet from 2000 to 2019.
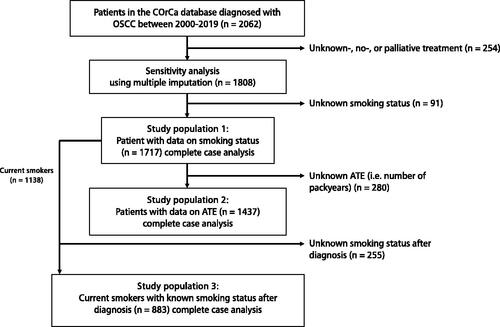
The included patients were divided into three study populations (). ‘Study population 1′ included all patients with known smoking status at diagnosis: never smokers (had never smoked), current smokers (smoked at diagnosis), and former smokers (quit smoking before the date of diagnosis). ‘Study population 2’ included patients from ‘study population 1’ with known ATE history prior to diagnosis. ATE was considered in two ways: (1) as ‘continuous ATE’ i.e. 0–183 PYs and (2) as ‘categorical ATE’, i.e. 0, 0x
30, 30
x
60,
60 PYs. ‘Study population 3’ included current smokers from ‘study population 1’ with known smoking status after diagnosis, i.e. smoking cessation at diagnosis. It was assumed that this smoking status covered the entire period between diagnosis to an event or censuring – thereby that the patients did not change smoking status multiple times after diagnosis.
Figure 1. Flowchart illustrating the selection process of patients from the Copenhagen Oral Cavity Squamous Cell Carcinoma (COrCa) database included in analyses of this study, and reasons for exclusions. ATE: accumulated tobacco exposure.
We included data on sex, age at diagnosis, year of diagnosis, smoking status (never-, former-, and current smoker), ATE (number of PYs smoked before diagnosis), 7th edition of UICC/AJCC stage (I, II, III and IV), T- and N-stage (T1, T2, T3, T4 and N0, N1, N2), tumor subsite (floor of mouth (FOM), oral tongue, gingiva, and ‘others’), excessive alcohol consumption (EAC, ever- or never-EAC), treatment method (surgery, surgery with adjuvant radiotherapy, and primary radiotherapy), and smoking cessation at diagnosis (yes, no, unknown). This information was retrospectively obtained by evaluating the medical records of the patients. EAC was defined as on average more than two units (males) and one unit (females) of alcohol a day, which is the current limits recommended by the Danish Health Authority and the U.S. Department of Health and Human Services [Citation42].
Date of diagnosis was registered as the detection date of OSCC in a biopsy. Patients with no event before the last follow-up date were censored at this date. OS was calculated as the time from date of diagnosis until death from any cause, and DFS as time from date of diagnosis until recurrence, new primary tumor, or death from any cause. In the COrCa database recurrence, both distant and local recurrences were registered and defined according to the Odense-Birmingham definition [Citation43].
Statistical analyses
Kaplan–Meier (KM) estimator was used to calculate the cumulative survival proportions (2- and 5-year OS and DFS) and to plot KM survival curves stratified by smoking status and categorical ATE. Log-rank tests were used to test for differences between the KM curves.
Chi-squared test or Kruskal-Wallis test were used depending on the outcome variable to test for differences between the groups with different smoking histories ( and Supplementary Table 1). Spearman rank correlation analysis was performed between smoking status and alcohol consumption to investigate possible collinearity.
Table 1. Baseline characteristics of the included patients (study population 1) with oral squamous cell carcinoma (OSCC) from the COrCa database diagnosed between 2000–2019 stratified by smoking status: never-, current and former smokers.
Based on cumulative Schoenfeld residual plots and log-minus-log curves, proportionality was met for the included variables, except for the following: UICC7 stage, categorical ATE, continuous age, and smoking cessation at diagnosis. Therefore, we used the UICC7 stage as a stratification factor rather than a predictor by allowing the baseline hazard function to differ (strata-function in R). The non-proportionality of categorical ATE (0x
30 PYs) motivated the analyses of a possible non-linear effect of continuous ATE. The continuous age variable was divided into 10 five-year intervals, which fulfilled the proportionality assumption. Based on the cumulative residual plots, the time-dependent covariate smoking cessation at diagnosis on survival estimates were divided into two intervals individually fulfilling the proportionality assumption (Supplementary Figure 1).
Continuous ATE was plotted as a function of age at diagnosis, and showed no linear relationship between the number of PYs and age at diagnosis, therefore indicating no obvious time-dependence of ATE (Supplementary Figure 2).
A possible non-linear relationship between continuous ATE and HR for OS and DFS were tested using restricted cubic spline (RCS) models, with an indicator variable corresponding to ever-smoking (yes/no) and adjusted for α: sex (binary variable), age at diagnosis (categorical variable with 10 five-year intervals), year of diagnosis (continuous integer variable), UICC7 stage (categorical variable), tumor subsite (categorical variable), and EAC (binary variable). We applied four knots placed according to previous literature [Citation44].
Multivariable Cox regression analyses were performed to evaluate the effect of smoking status and categorical ATE at diagnosis on HR for OS and DFS, assuming a linear relationship based on the previous RCS modeling of continues ATE, and adjusted for α. The effect of smoking cessation at diagnosis on the HR for OS and DFS were analyzed adjusting for β: same variables as in α as well as categorical ATE. Equivalent analyses were performed without adjustment for EAC (γ and δ), as it may be difficult to separate the effects of smoking and alcohol consumption, on the survival estimates ().
Table 2. Multivariable Cox regression analyses adjusted for the below mentioned variables on overall survival (OS) and disease-free survival (DFS) for patients with oral squamous cell carcinoma from the COrCa database.
In this study, only complete case analyses were performed. Analyses equivalent to the above-mentioned multivariable Cox regression analyses were performed after applying multiple imputation on the dataset (n = 1808), assuming data was missing at random (Supplementary Table 2).
In the interaction analyses, we looked for the presence of interactions between: (A) EAC and ATE, and (B) tumor subsite and ATE, on survival estimates. The interactions were evaluated based on effect sizes (visualize by plotting the models) and p-values for significant differences between the models - with and without the interaction term (tested with a partial likelihood test). Continuous ATE was included through a RCS model in the multivariable Cox regression analyses with interaction terms – allowing the effect of ever-smoking (yes/no) as well as the effect of continues ATE to depend on respectively, (A) EAC and (B) tumor subsite, and adjusted for α.
We applied a 5% significance level and use the term significant for statistically significant. The analyses were performed in R version 3.6.1.
Results
We included 1717 OSCC patients treated with curative intent and with known smoking status (study population 1) from the COrCa database ( and ). The median follow-up time was 6.7 years (IQR: 3.9–11.1 years). Data on ATE were available for 1437 of the included patients (study population 2) and were divided into the four categories of ATE (Supplementary Table 1). Current smokers had a larger ATE history compared to former smokers.
Among the included patients 62.8% were males. The largest proportion of never-smokers were females, while the largest proportion of current and former smokers were males, and the proportion of males increased with increasing categorical ATE. The median age at diagnosis of 63.7 years (IQR: 56.5–71.3 years). Current smokers were on average almost 10 years younger than never-smokers at diagnosis. A large fraction of current smokers had an ever-EAC and the fraction of patients with an ever-EAC increased with categorical ATE. Ever-/never-smoking and ever-/never-EAC was moderately correlated (rho = 0.34, p < 0.01). T-stage T1 or T2 and N-stage N0 were most common for all groups. The subsite oral tongue was more common for never- and former smokers, while FOM was more common for current smokers. With increasing categorical ATE the fraction of tumors in the FOM increased while the fraction of tumors in the oral tongue decreased. The majority of the patients in all groups were treated with surgery, but current smokers and patients with large ATE received adjuvant radiotherapy or primary radiotherapy more frequently than never-smokers.
The KM estimates for 5-year OS and DFS were 53.7% (95% CI: 49.8%–57.9%) and 43.9% (95% CI: 40.0–48.1%), respectively, for all the 1717 included patients in study population 1. KM curves of OS (log-rank: p < 0.0001) and DFS (log-rank: p = 0.0076) stratified by smoking status illustrated the inferior survival probability of current and former smokers compared to never-smokers (). The 2- and 5-year OS were significantly lower for current versus never-smokers and non-significantly lower for former versus never-smokers. The 5-year DFS was neither significantly different for current nor former smokers compared to never-smokers.
Figure 2. Kaplan–Meier (KM) curves for overall survival (OS) and disease-free survival (DFS). KM-curves for (A) OS and (B) DFS stratified by smoking status, and (C) OS and (D) DFS stratified by categorical ATE. The 2- and 5-year survival probability (in percent) with 95% confidence intervals are tabulated below.
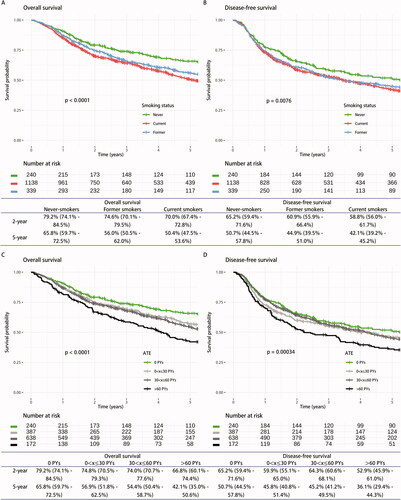
KM curves for OS (log-rank: p < 0.0001) and DFS (log-rank: p = 0.00034) stratified by categorical ATE, showed decreasing survival probability with increasing categorical ATE (). Notably, the survival probability of patients with 0x
30 and 30
x
60 PYs were almost the same in the whole follow-up period for both OS and DFS. The 5-year survival was significantly lower for patients with 30
x
60 (OS) and
60 (OS and DFS) PYs compared to never-smokers (i.e., 0 PYs).
We tested the possible non-linear relationship between continuous ATE and the survival estimates by using RCS models in multiple Cox regression analyses (Supplementary Figure 3). The HRs for OS and DFS increased almost linearly with increasing ATE in the interval 0-80 PYs. Significantly elevated HRs for survival estimates were identified for patients with ≥18 PYs (OS) and ≥70 PYs (DFS). These analyses therefore supported the assumption of a linear effect of ATE on the survival estimates.
In the multivariable Cox regression analyses (), current but not former smokers had significantly elevated HRs for OS and DFS, compared to never-smokers. However, when leaving out the adjustment for EAC also former smokers had significant elevated HR for OS. No significant differences in the survival estimates between former- and current smokers were found. The modeling of categorical ATE showed a significant elevated HR for OS with increasing categorical ATE and only patients with >60 PYs had a significantly elevated HR for DFS, compared to patients with 0 PYs. Furthermore, no significant differences in the survival estimates were observed between patients with 0x
30, 30
x
60, and
60 PYs.
Interestingly, 23.5% of the current smokers quit smoking at diagnosis, while 54.1% continued smoking after diagnosis (study population 3 with 22.4% missing values). A significantly unfavorable prognosis (HR for OS and DFS of approximately 2.3) was identified 3.5 (OS) and 2.5 (DFS) years after diagnosis and beyond, for patients who continued smoking after diagnosis compared to patients with smoking cessation at diagnosis ( and Supplementary Figure 4).
The sensitivity analysis showed similar results from all the equivalent multivariable Cox regression analyses on the data generated from multiple imputation (Supplementary Table 2).
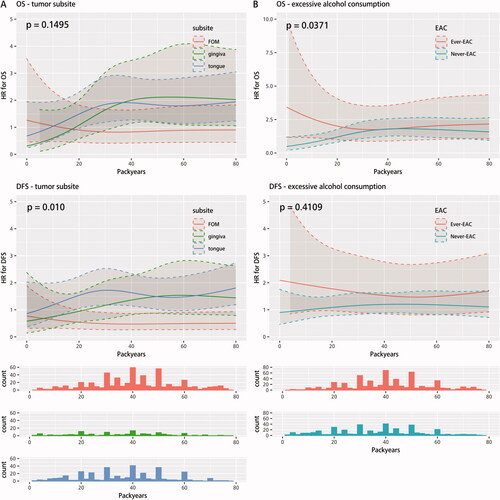
Furthermore, we analyzed a possible interaction between continuous ATE and tumor subsite on the survival estimates (). The HR for DFS of patients with a tumor located in the oral tongue (and gingiva) depended on the ATE, while no dependency was found for patients with a tumor located in the FOM. In the interval 20-60 PYs the HR for DFS was significantly elevated for patients with a tumor located in the oral tongue compared to FOM. Similar tendencies, but with overlapping confidence intervals, were found when analyzing the effect of tumor subsite on HR for OS. Finally, we considered a possible interaction between continuous ATE and EAC on the survival estimates (). In the interval of 0–5 PYs the HR for OS of patients with an ever-EAC was significantly higher than patients with a never-EAC. No significant difference was identified for the remaining ATE (5–80 PYs). Similar tendencies were observed for the effect of EAC on HR for DFS, however they did not differ significantly at any ATE.
Figure 3. Interaction analyses between smoking history (continuous ATE (i.e. number of PYs) included through a restricted cubic spline model and smoking status (yes/no)) and (A) tumor subsite (FOM, gingiva and oral tongue) and (B) excessive alcohol consumption (ever- and never-EAC) on survival estimates (OS and DFS) adjusted for α: sex, age at diagnosis, year of diagnosis, UICC7 stage, tumor subsite, and alcohol consumption. p-values for heterogeneity between multivariable Cox regression analyses (with and without an interaction term), were placed in the upper left corners of the plots. The patients with 0 Pys are not shown in the histograms. ATE: accumulated tobacco exposure. OS: overall survival; DFS: disease-free survival; FOM: floor of mouth; EAC: excessive alcohol consumption.
Discussion
In this study, we investigated the impact of smoking status and ATE on the survival of patients with OSCC. Most studies on this topic investigate the effect of being an ever- versus never-smoker while only some studies [Citation19–21,Citation24,Citation29] further differentiate between current and former smokers. The studies by Sharp et al. [Citation24] (n = 1469), Beynon et al. [Citation21] (oral cancer: n = 403), and Fang et al. [Citation20] (n = 198) found, consistent with our findings, a significant negative effect on the survival of being current (DSS, RFS, and OS) or former (OS) smoker compared to never-smoker, and no significant differences in the survival estimates between current- and former smokers. The distribution of applied treatment methods in our study was similar to the distribution applied in the study by Beynon et al. [Citation21], while cancer-directed surgery was less applied in the study by Sharp et al. [Citation24] and radiotherapy less applied in the study by Fang et al. [Citation20].
In contrast to our findings, several studies [Citation19,Citation26,Citation28,Citation29] found no significant effect of smoking on the survival estimates. The inconsistencies with the studies by Giraldi et al. [Citation19] (oral cancer: n = 1404) and Zanoni et al. [Citation28] (n = 2082) may be due to the adjustment for histopathological variables, the inclusion of patients with different ethnicities, and/or the inclusion of patients no matter their treatment method – if any. We only enrolled patients treated with curative intent in order not to overestimate the effect of smoking. Additionally, neither Giraldi et al. [Citation19] nor Zanoni et al. [Citation28] adjusted for tumor subsite or sex in their studies in contrast to us.
In the studies by Quinlan-Davidson et al. [Citation29] (n = 289) and Oh et al. [Citation26] (n = 3379) univariable analyses were performed, and they found, similar to the two aforementioned studies [Citation19,Citation28], no significant effects of ever-smoking compared to never-smoking on survival. A possible explanation for these inconsistencies with our results could be due to differences in treatment approach. A large fraction of patients in the study performed by Quinlan-Davidson et al. [Citation29] received radiotherapy (83.7%), in contrast to 38.7% in Oh et al. [Citation26] and 47.0% in our study. Notably, in both studies a larger fraction of patients had a tumor located to the oral tongue (51% [Citation29] and 66% [Citation26]) and a lower fraction to the FOM (15% [Citation29] and 10% [Citation26]) in comparison to our study (tongue: 35.5% and FOM: 40.2%).
Similar to Fazel et al. [Citation30], we found an effect of altering smoking habits at the time of diagnosis on survival; however, only significant after approximately three years. These analyses were associated with some uncertainty as an underlying assumption was that the patients did not change smoking status multiple times after diagnosis. However, these results emphasize the critical role of tobacco smoking cessation interventions for patients with OSCC.
Smoking status at diagnosis may not be the best parameter for evaluating the impact of smoking on clinical outcomes, as these variables do not take the total amount of smoked tobacco into account. We therefore included analyses on the effect of ATE. Although ATE is a more adequate measure for evaluating the impact of smoking history, it should be noted that this data was obtained retrospectively.
Previous studies [Citation19,Citation22,Citation29] on the impact of ATE on the survival of patients with OSCC were sparse and had different conclusions. Quinlan-Davidson et al. [Citation29] found no significant effect of having smoked >20 PYs compared to <20 PYs on the HR for OS. They only included 289 patients all treated with surgery and intensity-modulated radiotherapy and performed univariable analysis, which could be some of the reasons for the inconsistencies with our results. Furthermore, Kawakita et al. [Citation22] analyzed smoking in a dose-dependent fashion and found a significantly elevated HR for OS for patients with 60 and 30
x
60 PYs and a significantly elevated HR for DSS for patients with 30
x
60 PYs, both compared to patients with 0
x
30 PYs. We did not use patients with 0
x
30 PYs as reference group and assumed that the finding of a worse prognosis of patients with 0 PYs was because of the few included patients (n = 222) and/or the adjustment for performance score and treatment method, and/or no adjustment for tumor subsite and age at diagnosis. We did not adjust for the treatment method because the choice of treatment method was based on, e.g. the UICC7 stage.
We found the subsite FOM to be much more common for current smokers than for never-smokers. This was compatible with previous findings by Schmidt et al. [Citation6], who found a strong association between a history of smoking and the occurrence of carcinomas involving FOM and posterior tongue. Interestingly, we identified a significant (p = 0.01) interaction between ATE and subsite on HR for DFS. Kawakita et al. [Citation22] also looked for the presence of an interaction between subsite and ATE on HR for OS and found no significant (p = 0.90) interaction. However, they likewise found a tendency of a decreasing survival for tongue cancer patients with increasing categorical ATE. Previous studies [Citation27,Citation45] on the effect of smoking on the survival of patients with oral tongue cancer have been conflicting. Bachar et al. [Citation27] analyzed the survival of oral tongue cancer patients with and without known risk factors and found no difference in local and regional control as well as OS. El-Husseiny et al. [Citation45] found a significantly worse OS and DFS of smoking oral tongue cancer patients compared to nonsmoking patients. Our results indicated that the tumor subsite modulated the effect of ATE on survival.
The interaction between EAC and ATE on survival estimates were also investigated and showed no convincing interacting effect, despite the low p-value (OS: p = 0.04). A previous study [Citation46] on the effect of smoking and alcohol consumption on the survival of patients with oral cancer has concluded that smoking and drinking patients have a significantly higher HR for OS and DSS compared to non-drinking nonsmoking patients. It therefore remains unanswered whether smoking and EAC have an interacting effect on the survival of patients with OSCC.
Notable limitations: First, the relatively low number of former and never-smokers compared to current smokers weakened somewhat the power of the comparisons. Second, ATE history was self-reported and thus subjected to recall bias as well as bias from the subjective interpretation of treating physicians. Potentially resulting in under-/overestimation of the true impact of smoking history on survival. Third, data on cause-of-death, comorbidities, and histopathological variables was not currently accessible for the patients. It was therefore not possible to calculate DSS or adjust for additional potential confounders.
Methodological strengths: First, the healthcare system in Denmark provides the population with free access to all diagnostics and treatments, financed by general taxes. This entailed that patients were not selected, and treatment was initiated when indicated, irrespective of e.g. the patient’s economy and insurance. Second, analyses were based on variables describing different aspects of smoking history, thereby improving the modeling of the effect of smoking on survival.
Conclusion
In conclusion, tobacco smoking (assessed as smoking status and ATE) was associated with an inferior survival (OS and DFS) among patients with OSCC. The unadjusted KM-curves showed inferior survival probability of smokers (both former and current smokers) and patients with increasing ATE (0x
30, 30
x
60,
60 PYs), compared to never-smokers (with 0 PYs). Based on fully adjusted multivariable Cox regression analyses, elevated HRs for OS and DFS were significant for current but not former smokers, compared to never-smokers. Additionally, multivariable Cox regression analyses on ATE (analyzed non-linearly (as a continuous variable) and linearly (as a categorical variable)) showed elevated HR for OS with increasing PYs and elevated HR for DFS only for patients with >60 PYs, compared to never-smokers. An unfavorable long-term prognosis was evident after >3.5 (OS) and >2.5 (DFS) years from diagnosis for patients who continued smoking compared to patients with smoking cessation at diagnosis. Finally, the interaction analyses indicated that the impact of ATE on survival (OS and DFS) for patients with OSCC may depend on tumor subsite and/or the alcohol consumption.
Supplemental Material
Download MS Word (21.2 KB)Supplemental Material
Download MS Word (25.4 KB)Supplemental Material
Download MS Word (61.6 KB)Supplemental Material
Download MS Word (39.9 KB)Supplemental Material
Download MS Word (96.4 KB)Supplemental Material
Download MS Word (930.1 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
Data are available from the corresponding author upon request, however transfer of data requires specific approval from the Danish authorities.
Additional information
Funding
References
- Ali J, Sabiha B, Jan HU, et al. Genetic etiology of oral cancer. Oral Oncol. 2017;70(2017):23–28.
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2021;71(3):209–249.
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Simard EP, Torre LA, Jemal A. International trends in head and neck cancer incidence rates: Differences by country, sex and anatomic site. Oral Oncol. 2014;50(5):387–403.
- Karnov KKS, Grønhøj C, Jensen DH, et al. Increasing incidence and survival in oral cancer: a nationwide Danish study from 1980 to 2014. Acta Oncol. 2017;56(9):1204–1209.
- Schmidt BL, Dierks EJ, Homer L, et al. Tobacco smoking history and presentation of oral squamous cell carcinoma. J Oral Maxillofac Surg. 2004;62(9):1055–1058.
- Llewelyn J, Mitchell R. Smoking, alochol and oral cancer in South East Scotland: a 10-year experience. Br J Oral Maxillofac Surg. 1994;32(3):146–152.
- Morse DE, Psoter WJ, Cleveland D, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Causes Control. 2007;18(9):919–929.
- U.S. Department of Health and Human Services. How tobacco smoke causes disease: the biology and behavioral basis for smoking-attributable disease. A Report of the Surgeon General. 2010. p. 792.
- Rothman K, Keller A. The effect of joint exposure to alcohol and tobacco on risk of cancer of the mouth and pharynx. J Chronic Dis. 1972;25(12):711–716.
- Mello FW, Melo G, Pasetto JJ, et al. The synergistic effect of tobacco and alcohol consumption on oral squamous cell carcinoma: a systematic review and Meta-analysis. Clin Oral Investig. 2019;23(7):2849–2859.
- Jaber MA, Porter SR, Gilthorpe MS, et al. Risk factors for oral epithelial dysplasia-the role of smoking and alcohol. Oral Oncol. 1999;35(2):151–156.
- Gatta G, Botta L, Sánchez MJ, et al. Prognoses and improvement for head and neck cancers diagnosed in Europe in early 2000s: the EUROCARE-5 population-based study. Eur J Cancer. 2015;51(15):2130–2143.
- Schwam ZG, Judson BL. Improved prognosis for patients with oral cavity squamous cell carcinoma: analysis of the national cancer database 1998-2006. Oral Oncol. 2016;52:45–51.
- Pytynia KB, Grant JR, Etzel CJ, et al. Matched-pair analysis of survival of never smokers and ever smokers with squamous cell carcinoma of the head and neck. J Clin Oncol. 2004;22(19):3981–3988.
- Fortin A, Wang CS, Vigneault É. Influence of smoking and alcohol drinking behaviors on treatment outcomes of patients with squamous cell carcinomas of the head and neck. Int J Radiat Oncol Biol Phys. 2009;74(4):1062–1069.
- Fazel A, Quabius ES, Fabian A, et al. The influence of smoking and Co-morbidity on dose achievement in primary or adjuvant radio(chemo)therapy in head and neck squamous cell carcinoma (HNSCC). Front Oncol. 2020;10:398.
- Farshadpour F, Kranenborg H, Calkoen EVB, et al. Survival analysis of head and neck squamous cell carcinoma: influence of smoking and drinking. Head Neck. 2011;33(6):817–823.
- Giraldi L, Leoncini E, Pastorino R, et al. Alcohol and cigarette consumption predict mortality in patients with head and neck cancer: a pooled analysis within the international head and neck cancer epidemiology (INHANCE) consortium. Ann Oncol. 2017;28(11):2843–2851.
- Fang QG, Shi S, Liu FY, et al. Squamous cell carcinoma of the oral cavity in ever smokers: a matched-pair analysis of survival. J Craniofac Surg. 2014;25(3):934–937.
- Beynon RA, Lang S, Schimansky S, et al. Tobacco smoking and alcohol drinking at diagnosis of head and neck cancer and all-cause mortality: results from head and neck 5000, a prospective observational cohort of people with head and neck cancer. Int J Cancer. 2018;143(5):1114–1127.
- Kawakita D, Hosono S, Ito H, et al. Impact of smoking status on clinical outcome in oral cavity cancer patients. Oral Oncol. 2012;48(2):186–191.
- Bundgaard T, Bentzen SM, Wildt J. The prognostic effect of tobacco and alcohol consumption in intra-oral squamous cell carcinoma. Eur J Cancer Part B Oral Oncol. 1994;30(5):323–328.
- Sharp L, McDevitt J, Carsin AE, et al. Smoking at diagnosis is an independent prognostic factor for cancer-specific survival in head and neck cancer: findings from a large, population-based study. Cancer Epidemiol Biomarkers Prev. 2014;23(11):2579–2590.
- Lee SU, Moon SH, Choi SW, et al. Prognostic significance of smoking and alcohol history in young age oral cavity cancer. Oral Dis. 2020;26(7):1440–1448.
- Oh LJ, Asher R, Veness M, et al. Effect of age and gender in non-smokers with oral squamous cell carcinoma: multi-institutional study. Oral Oncol. 2021;116:105210.
- Bachar G, Hod R, Goldstein DP, et al. Outcome of oral tongue squamous cell carcinoma in patients with and without known risk factors. Oral Oncol. 2011;47(1):45–50.
- Zanoni DK, Montero PH, Migliacci JC, et al. Survival outcomes after treatment of cancer of the oral cavity (1985–2015). Oral Oncol. 2019;90:115–121.
- Gunn GB, Johnson FM, Skinner H, et al. Outcomes of oral cavity cancer patients treated with surgery followed by postoperative intensity modulated radiation therapy. Oral Oncol. 2017;72:90–97.
- Fazel A, Quabius ES, Gonzales-Donate M, et al. Alteration of smoking habit at time of first diagnosis influences survival of patients with HNSCC. Mol Clin Oncol. 2020;13(5):1–10.
- Gerude MF, Dias FL, De Farias TP, et al. Predictors of postoperative complications, prolonged length of hospital stay, and short-term mortality in elderly patients with malignant head and neck neoplasm. ORL J Otorhinolaryngol Relat Spec. 2014;76(3):153–164.
- Vandersteen C, Dassonville O, Chamorey E, et al. Impact of patient comorbidities on head and neck microvascular reconstruction. A report on 423 cases. Eur Arch Otorhinolaryngol. 2013;270(5):1741–1746.
- Harris CC, Hollstein M. Influence of cigarette smoking on the efficacy of radiation therapy in head and neck cancer. N Engl J Med. 1993;329(18):1318–1327.
- Do KA, Johnson MM, Doherty DA, et al. Second primary tumors in patients with upper aerodigestive tract cancers: joint effects of smoking and alcohol (United States). Cancer Causes Control. 2003;14(2):131–138.
- Silverman S, Gorsky M, Greenspan D. Tobacco usage in patients with head and neck carcinomas: a follow-up study on habit changes and second primary oral/oropharyngeal cancers. J Am Dent Assoc. 1983;106(1):33–35.
- Chang SS. Re: smoking cessation: a report of the surgeon general. J Urol. 2020;204(2):384–384.
- Janjigian YY, McDonnell K, Kris MG, et al. Pack-years of cigarette smoking as a prognostic factor in patients with stage IIIB/IV nonsmall cell lung cancer. Cancer. 2010;116(3):670–675.
- Saquib N, Stefanick ML, Natarajan L, et al. Mortality risk in former smokers with breast cancer: pack-years vs. smoking status. Int J Cancer. 2013;133(10):2493–2497.
- Nagle CM, Bain CJ, Webb PM. Cigarette smoking and survival after ovarian cancer diagnosis. Cancer Epidemiol Biomarkers Prev. 2006;15(12):2557–2560.
- DAHANCA & Onkologi D selskab for HH. Behandling af Planocellulaert Karcinom i Mundhulen Nationale Retningslinjer [Treatment Plan of Oral Cavity Squamous Cell Carcinoma, National Guidelines]. Udg. 3 Version 8. marts 2016. Available from: www.dahanca.dk
- Jensen JS, Jakobsen KK, Mirian C, et al. The Copenhagen oral cavity squamous cell carcinoma database: protocol and report on establishing a comprehensive oral cavity cancer database. Clin Epidemiol. 2019;11:733–741.
- UDSA. Dietary guidelines for Americans, 2020-2025. 9th Edition. Am J clin nutr. 2020. 34(1):121–123.
- Rohde M, Rosenberg T, Pareek M, et al. Definition of locally recurrent head and neck squamous cell carcinoma: a systematic review and proposal for the Odense–Birmingham definition. Eur Arch Otorhinolaryngol. 2020;277(6):1593–1599.
- Croxford R. Restricted cubic spline regression: a brief introduction. Toronto Inst Clin Eval Sci. Paper 5621. 2016.
- El-Husseiny G, Kandil A, Jamshed A, et al. Squamous cell carcinoma of the oral tongue: an analysis of prognostic factors. Br J Oral Maxillofac Surg. 2000;38(3):193–199.
- Bao X, Liu F, Chen Q, et al. Propensity score analysis exploring the impact of smoking and drinking on the prognosis of patients with oral cancer. Head Neck. 2020;42(8):1837–1847.
