Abstract
Background
Patients with potentially curable esophageal cancer can be treated with neo-adjuvant chemoradiotherapy followed by surgery or definitive chemoradiotherapy with curative intent. For frail older patients choosing the appropriate oncological treatment can be difficult, and data on geriatric deficits as determinants of treatment outcomes are not yet available.
Objectives
To describe the prevalence of geriatric deficits and to study their association with treatment discontinuation and mortality in older patients with potentially curable esophageal cancer.
Material and Methods
A cohort study was conducted in a Dutch tertiary care hospital including patients aged ≥70 years with primary stage I-IVA esophageal cancer. Geriatric screening and assessment data were collected. Outcomes were treatment discontinuation and one year all-cause mortality.
Results
In total, 138 patients with curable esophageal cancer were included. Mean age was 76.1 years (standard deviation 4.7), 54% had clinical stage III and 24% stage IVA disease. Most patients received neo-adjuvant chemoradiotherapy and surgery (41%), 32% definitive chemoradiotherapy and 22% palliative radiotherapy. Overall, one year all-cause mortality was 36%. Geriatric screening and assessment was performed in 94 out of 138 patients, of which 60% was malnourished, 20% dependent in Instrumental Activities of Daily Living (IADL) and 52% was frail. Malnutrition was associated with higher mortality risk (Hazard Ratio, 3.2; 95% Confidence Interval, 1.3–7.7)) independent of age, sex and tumor stage. Seventy-six out of 94 patients were treated with chemoradiotherapy, of which 23% discontinued treatment. Patients with IADL dependency and Charlson Comorbidity Index ≥1 discontinued treatment more often.
Conclusion
All-cause mortality within one year was high, irrespective of treatment modality. Treatment discontinuation rate was high, especially in patients treated with definitive chemoradiotherapy. Geriatric assessment associates with outcomes in older patients with esophageal cancer and may inform treatment decisions and optimization in future patients, but more research is needed to establish its predictive value. Trial registration: The study is retrospectively registered at the Netherlands Trial Register (NTR), trial number NL8107. Date of registration: 22-10-2019.
Background
Esophageal cancer mainly affects older patients. Around 60% of newly diagnosed patients are aged ≥65 years [Citation1]. Although older patients are heterogenous with respect to the presence of geriatric deficits [Citation2], it is unclear how prevalent these deficits are in this patient group and their association with outcomes is also unknown.
Despite the majority of patients being older, treatment guidelines for esophageal cancer are not well defined. Current guidelines are based on clinical trials in younger and fitter patients. Neo-adjuvant chemoradiotherapy (CRT) followed by surgery is the standard treatment approach for patients with potentially curable esophageal cancer, with 2-year survival rates of 62%–70% [Citation3,Citation4]. Patients with non-metastatic disease who are not suitable for surgery can be treated with definitive chemoradiation (dCRT). Although dCRT is also a treatment with curative intent, survival is worse compared to the combination treatment of CRT plus surgery, with a 2-year survival of 30%–50% [Citation5–8]. For frail older patients this is often the treatment of choice [Citation9]. When curative therapy is not an option, other therapies include palliative radiotherapy, systemic therapy, or no anti-tumor therapy.
Older age and geriatric deficits are associated with severe chemotherapy toxicity and discontinuation [Citation10]. Treatment toxicity may lead to more hospital admissions, functional dependency and reduced quality of life. Furthermore, early treatment discontinuation results in suboptimal cancer treatment. A geriatric assessment identifies geriatric deficits and might thereby help select patients at high risk of toxicity, discontinuation and (early) mortality. However, currently limited data on geriatric deficits as determinants of treatment discontinuation and mortality after chemoradiotherapy for esophageal cancer are available [Citation11].
Therefore, the aims of the present study are to describe the prevalence of deficits in geriatric assessment and study their association with treatment discontinuation and mortality in a Dutch cohort of older patients with potentially curable esophageal cancer.
Material and methods
Study design, setting and procedures
A single-center cohort study was conducted in the upper gastrointestinal (GI) oncology outpatient clinic at Leiden University Medical Center (LUMC). Care for patients with esophageal cancer is centralized at the LUMC for four surrounding hospitals that refer all patients who may be candidate for curative treatment to this tertiary center. Patients were seen by a multidisciplinary team consisting of surgeons, medical oncologists, radiation oncologists and gastroenterologists. In 2015 a short geriatric screening was implemented in the routine clinical care pathway for patients aged ≥70 years. The screening was performed by a trained nurse and consisted of the Geriatric 8 (G-8) questionnaire [Citation12] and Six-Item Cognitive Impairment Test (6CIT) [Citation13]. Patients were referred to the geriatric outpatient clinic for a geriatric assessment (GA) when the screening was abnormal, as described below. GA results, including recommended interventions for pretreatment optimization and treatment modifications, were discussed with treating physicians during a multidisciplinary team meeting, and discussed afterwards with the patient. Data collection for this study was in the context of the Triage Elderly Needing Treatment (TENT) study [Citation14], a prospective cohort study that is embedded in the routine care pathway. As part of this study patients not referred for GA at the geriatric outpatient clinic were contacted before treatment initiation for informed consent and GA by telephone. This consisted of the same tests assessed by a researcher and GA results were not discussed with treating physicians. The study was approved by the Medical Ethics Committee (ID number NL53575.058.15) at the LUMC and a ‘certificate of no objection’ was issued for data collection of patients not included in the study. More details on the design and rationale of the care pathway and TENT study were described elsewhere [Citation14].
Patients
Patients 70 years or older with potentially curable, clinical stage I-IVA (according to the American Joint Committee on Cancer Staging Form Supplement, eighth edition) epithelial esophageal cancer between June 2015 and June 2019 at the upper GI oncology outpatient clinic were consecutively included. Patients with recurrent esophageal cancer, clinical stage I cancer treated with endoscopic resection, clinical stage IVB cancer, incomplete clinical staging and patients that were treated elsewhere were excluded.
Data collection
Digital patient files were reviewed to collect the following data: age, sex, comorbidity, medication use, body mass index (BMI), smoking and alcohol status and history, WHO performance score (WHO PS), histopathological cancer type, clinical stage of disease, treatment and course. If uncertainty about the cT or cN category resulted in multiple staging options, the highest stage was chosen. Radiotherapy and chemotherapy toxicity was defined by the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events version 5.0 (CTCAE).
Geriatric screening and assessment
G-8 score ranges from 0 to 17, score ≤14 was considered abnormal. 6CIT ranges from 0 to 28, score >7 was considered abnormal. Abnormal geriatric screening was defined if at least one test was abnormal. In the context of the TENT study, a GA was administered in all patients with geriatric screening, regardless of the screening results. Data were collected according to the four geriatric domains: the somatic, psychological, functional and social domains. Patients who scored abnormal on at least two geriatric domains were classified as frail. A domain was considered abnormal if at least one individual test of the corresponding domain was scored abnormal. The somatic domain consisted of comorbidity, polypharmacy and malnutrition assessed by the Mini Nutritional Assessment Short Form (MNA-SF®; range 0 to 14, cutoff score ≤11) [Citation15]. Comorbidities were assessed by the Charlson Comorbidity index (CCI) [Citation16]. No points were assigned to the ‘solid tumor’ category if no other tumors were present as this was considered to be the main diagnosis. Therefore, presence of comorbidity was defined as CCI ≥1. The somatic domain was abnormal in case of CCI ≥1, number of medications ≥5 or MNA-SF® ≤11. The psychological domain contained history of delirium, dementia and cognitive impairment according to the 6CIT. The functional domain included information on fall incidents in the past six months, institutionalization and functional dependency by the Katz Activities of Daily Living (ADL) questionnaire (range 0 to 6; cutoff score ≥2) [Citation17], and Lawton Instrumental Activities of Daily Living (IADL) (range 0 to 5 for men, cutoff score ≤4; range 0−8 for women, cutoff score ≤7) [Citation18]. The social domain was assessed by asking about the current living situation and was considered abnormal when patients lived alone.
Oncological treatment
In the Netherlands, neo-adjuvant CRT plus surgery is the standard treatment approach when the treatment intent is curative. The vast majority of patients that are fit for surgery are also fit for CRT. When patients are unfit for surgery dCRT is offered. Patients that have contraindications for CRT can be treated with surgery alone. Neo-adjuvant CRT consisted of a 5-week schedule of 23 fractions of 1.8 Gray (Gy) external beam radiotherapy and concurrent chemotherapy carboplatin (area under the curve (AUC) 2) and paclitaxel (50 mg/m2). Esophageal resection was performed using the transthoracic or transhiatal approach, depending on the location of the tumor. dCRT consisted of a 6-week schedule of 28 fractions of 1.8 Gy and concurrent chemotherapy carboplatin (AUC 2) and paclitaxel (50 mg/m2). Palliative external beam radiotherapy consisted of 20 fractions of 2.5 Gy, 13 fractions of 3 Gy, 5 fractions of 4 Gy or 1 fraction of 8 Gy.
Outcomes
Main outcomes were one year all-cause mortality and treatment discontinuation. Mortality data were extracted from digital patient files. Treatment discontinuation was defined as not completing the number of radiotherapy fractions, chemotherapy courses, or surgery that were planned at initiation.
Statistical methods
Normal and skewed distributed continuous data are presented as mean with standard deviation (SD) or median with interquartile range (IQR). Categorical data are presented as numbers with percentages. GA results were dichotomized. Differences in patient characteristics were assessed using the independent samples t-test for normally distributed data, Mann-Whitney U test for skewed data and chi-square test or Fisher’s exact test for categorical data. Differences in outcomes were assessed using chi-square test. Length of follow-up was calculated as the time from treatment initiation until death or censoring at the end of follow-up and was compared using the log-rank test. Cox regression analyses were performed to study the association between baseline characteristics and one year mortality. The multivariable associations were adjusted for age and sex (model 1), and for age, sex and tumor stage (model 2). Hazard ratios (HR) and adjusted hazard ratios (aHR) with 95% confidence intervals (CI) were calculated. P-value of <0.05 was considered statistically significant. Missing data are addressed in the table legends. Analyses were performed using SPSS software version 25.
Results
During the inclusion period, 197 patients aged 70 years or older, diagnosed with primary esophageal cancer had a consultation at the upper GI oncology outpatient clinic. Out of 197 patients, 138 patients had stage I–IVA cancer and were not treated with endoscopic resection (Supplemental Figure 1). shows their baseline characteristics. Mean age was 76.1 years (SD 4.7) and 103 (74.6%) were men. Seventy-five patients had clinical stage III (54.3%) and 33 had stage IVA cancer (23.9%). Patients were treated with neo-adjuvant CRT plus surgery (n = 56 (40.6%)), dCRT (n = 44 (31.9%)) or palliative radiotherapy (n = 30 (21.7%)). Geriatric screening was performed in 94 out of 138 patients (68.1%). The main reasons for not performing geriatric screening were upfront palliative treatment intention (n = 19) because of bad performance status (WHO ≥3), severe comorbidity or personal preference; or staff shortage (n = 23). Patients with geriatric screening and assessment had more clinical stage IVA disease, were less often treated with palliative radiotherapy and more often with dCRT, compared to patients without.
Figure 1. Association between geriatric deficit and chemoradiotherapy discontinuation. Percentage of patients that discontinued chemoradiotherapy according to geriatric deficit. N depicts the number of patients with indicated geriatric deficit. Percentage that discontinued chemoradiotherapy is compared between patients without geriatric deficits and patients with geriatric deficits, calculated with the χ2 test. *p < 0.05. ADL: Activities of Daily Living; CCI: Charlson Comorbidity Index; G-8: Geriatric 8; IADL: Instrumental Activities of Daily Living.
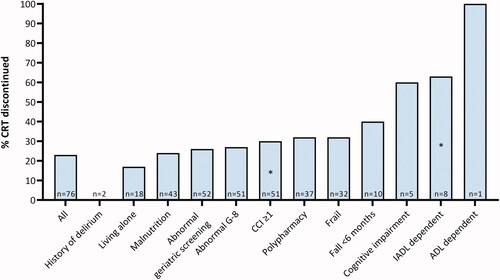
Table 1. Baseline characteristics.
Geriatric deficits
shows the results of geriatric assessment in patients with geriatric screening stratified for treatment modality. Sixty-eight patients (72.3%) had an abnormal geriatric screening consisting for 66 (70.2%) of an abnormal G-8 and 10 (11.0%) of an abnormal 6CIT score. All patients lived at home, with 24 living alone (25.8%). Fifty-six patients (60.2%) were malnourished. Sixteen patients (18.0%) recently fell and 16 patients (20.3%) were IADL dependent. Forty-five (51.7%) patients classified as frail. Overall, patients treated with dCRT or palliative radiotherapy had more geriatric deficits and were more often frail compared to patients treated with neo-adjuvant CRT plus surgery.
Table 2. Prevalence of geriatric deficits in patients with geriatric screening and in patients with different treatment modalities.
One year all-cause mortality
One year all-cause mortality of 138 patients with curable esophageal cancer was 36.2% with a mean follow-up time after 1 year of 10.5 months (95% CI, 9.8–11.2). One year mortality of 94 patients with geriatric screening was 37.2% and mean follow-up time was 10.7 months (95% CI, 10.0–11.5). Within this group, 1-year mortality rate was 30.8% in patients treated with neo-adjuvant CRT plus surgery, 32.4% in patients treated with dCRT, 64.3% in patients that received palliative radiotherapy and 50% in patients that received no anti-tumor treatment (). Mean follow-up was 11.4 months (95% CI, 10.5–12.2) for neo-adjuvant CRT plus surgery, 10.2 months (95% CI, 9.2–11.2) for dCRT, 8.9 months (95% CI, 6.3–11.5) for palliative radiotherapy and 9.4 months (95% CI, 5.7–13.0) for no anti-tumor treatment. Mortality rates and mean follow-up time for patients without geriatric screening are shown in Supplementary Table 1.
Table 3. Treatment outcomes in patients with geriatric screening who received chemoradiotherapy plus surgery or definitive chemoradiotherapy.
Association patient characteristics and one year all-cause mortality
shows the results of Cox regression analyses in patients with geriatric screening. Multivariable analysis showed a significant association with a higher risk for one year all-cause mortality for squamous cell carcinoma (aHR, 3.5; 95% CI 1.6–7.5) and malnutrition (aHR, 3.2; 95% CI 1.3–7.7) independent of age, sex and tumor stage.
Table 4. Association between patient characteristics and one year all-cause mortality in patients with geriatric screening (n = 94).
Treatment outcomes of patients with geriatric screening
Treatment details and outcomes of patients with geriatric screening who received chemoradiation are depicted in . Seventy-six patients were treated with chemoradiotherapy. Of the 73 patients with complete data, 17 patients (23.3%) discontinued treatment. Fourteen out of 73 patients (19.2%) discontinued chemotherapy, 2 patients (2.7%) discontinued chemo- and radiotherapy and in 1 patient (1.4%) the planned surgery was not performed. Chemotherapy discontinuation was caused by grade ≥3 toxicity in 12 patients and grade 2 toxicity in 2 patients. In both patients that discontinued chemo- and radiotherapy it was caused by grade ≥3 toxicity, of whom one patient had a grade 5 neutropenic sepsis and esophagitis. The reason for not undergoing surgery after chemoradiotherapy completion was personal preference. Overall, 45 patients (61.6%) experienced grade ≥3 toxicity. Patients treated with dCRT discontinued treatment more often (n = 11, 32.4%) compared to patients treated with neo-adjuvant CRT plus surgery (n = 6, 15.4%; p = 0.087). Of patients that discontinued treatment 41% died within one year, compared to 27% of patients that completed treatment. Fourteen patients were treated with palliative radiotherapy. One patient (7.7%) discontinued treatment, as he died of tumor progression. One patient treated with 39 Gray palliative radiotherapy experienced grade ≥3 toxicity.
Geriatric deficits and treatment discontinuation
IADL dependent patients discontinued chemoradiotherapy more often (62.5%) compared to patients that were IADL independent (20.0%; p = 0.02), as for patients with CCI ≥1 (30%) compared to patients with CCI 0 (8.7%; p = 0.05). Compared to patients with normal cognitive function, patients with cognitive impairment discontinued chemoradiotherapy more often (20.6% vs. 60%, p = 0.08). Also, frail patients discontinued chemoradiotherapy more frequently (32.3%) compared to fit patients (15.8%, p = 0.11), as for patients with polypharmacy (32.4%) compared to patients without polypharmacy (13.9%, p = 0.06) ().
Discussion
The main findings of the present study are threefold. First, geriatric deficits are highly prevalent in patients with curable esophageal cancer. Second, all-cause mortality within one year was high, irrespective of treatment modality and malnutrition was associated with higher mortality risk. Third, chemoradiotherapy resulted in high treatment discontinuation rates and occurred more often in patients with IADL dependency and comorbidity.
In the present study, the majority of patients was malnourished and frail. One out of four patients lived alone and twenty percent was IADL dependent. Only 1% of all patients referred to the outpatient clinic was institutionalized. Until now, data on geriatric assessment in older patients with esophageal cancer are very limited. A review by van Deudekom et al. concluded that 14%–67% of included patients were functionally impaired and 5%–23% did not have a partner [Citation11]. Wang et al. performed a GA before CRT initiation in 46 older patients with squamous cell carcinoma [Citation19]. Seventy percent was at risk for malnutrition, 39% IADL dependent and 10% ADL dependent. The prevalence of malnutrition and living alone in the present study was in line with van Deudekom and Wang et al. IADL and ADL dependency in the present study was lower compared to the study by Wang et al. Since other studies reported functional status as ECOG PS or KPS and no data on frailty is available, these results cannot be compared. The proportion of institutionalized patients in our cohort reflects the Dutch older population since only 3% of people older than 65 years were institutionalized in 2021 [Citation20].
Overall one year mortality rates were high: 37% irrespective of treatment and 32% in patients treated with CRT. In general, lower mortality rates are reported in older patients treated with neo-adjuvant CRT and surgery, compared to dCRT. Cooper et al. found a one year mortality rate of 17% in 65 patients aged ≥70 years treated with the CROSS regimen and surgery, similar to the CROSS trial [Citation21]. Other studies on various CRT regimes found one year mortality rates of 5–60% [Citation4,Citation8,Citation22–26]. The findings in our study are comparable with previous studies, except for the higher mortality rate in patients that had geriatric screening and were treated with neo-adjuvant CRT plus surgery. In the present study, malnutrition was associated with higher mortality rates independently of age, sex and tumor stage. This is in line with previous results in other tumor types [Citation27]. Our results suggest that besides malnutrition, mainly tumor characteristics and palliative treatment modality contributed to a higher mortality risk.
In the present cohort, treatment discontinuation rate was 23%, and patients with IADL dependency and comorbidity discontinued chemoradiotherapy more often. Rates reported in previous studies vary between 3–58% [Citation8,Citation21,Citation23,Citation24,Citation28–30], depending on treatment regimens, applied definitions and included patients. Overall, discontinuation rates are higher in patients treated with dCRT or old age compared to treatment with neo-adjuvant CRT plus surgery or young age. None of these studies described data on geriatric assessment. Treatment discontinuation rates in our study are in line with previous studies. Moreover, the prevalence of grade ≥3 chemoradiotherapy toxicity in the current study was high (61%), which also corresponds to rates reported in other studies between 22% and 74% [Citation8,Citation21,Citation23,Citation24,Citation29,Citation31,Citation32].
The differences in outcomes between the chemoradiotherapy groups should be interpreted with caution, because they are confounded by indication. This hampers conclusions about the efficacy of different treatment modalities. Yet, the current study shows interesting results. Treatment discontinuation and toxicity rates were higher in patients treated with dCRT. This is most likely explained by confounding by indication: patients selected for dCRT were often unfit for neo-adjuvant CRT plus surgery. The higher prevalence of geriatric deficits in patients treated with dCRT supports this, and therefore suggests that current selection of patients for dCRT instead of CRT plus surgery based on geriatric deficits is justified. Moreover, mortality rates were comparable between CRT groups, despite the differences in geriatric deficits and better prognosis after neo-adjuvant CRT followed by surgery in previous studies.
Geriatric assessment identifies unrecognized health issues in older patients. Research in other oncology fields has shown that both treatment discontinuation and mortality are associated with geriatric deficits [Citation10,Citation33]. The results of the present study mark the importance of geriatric assessment. As chemoradiotherapy is often applied in older patients, it is of importance to recognize patients at high risk of treatment toxicity and discontinuation. The higher rates of severe treatment toxicity and discontinuation in patients treated with dCRT in the current study suggest that this treatment was not suitable for some patients and selection can be improved. Furthermore, these high rates may reflect a sharp decrease in quality of life and functional independence, in a group of patients with high risk of mortality. These data may be of importance in shared decision making of treatment choice in older patients with frailty. Due to the observational character of this study, it is not possible to conclude whether geriatric screening and assessment will improve outcomes. However, two recent randomized controlled trials showed that GA-based interventions decreased the incidence of chemotherapy toxicity [Citation34] and discontinuation [Citation35] and improved quality of life [Citation35] in other tumor types. In this and future research it is difficult to distinguish prognostication from optimization since it is unethical to not apply GA-based intervention for geriatric deficits while the effects of interventions are mostly multifactorial themselves and hard to differentiate. Moreover, randomized controlled trials are challenging to conduct due to the heterogeneity of the older population and complexity of interventions.
The current study has several limitations. First, this was a single center study with centralized care for a cluster of five hospitals. Although care followed international guidelines, generalizability beyond this cluster needs to be studied. Second, a large proportion of patients had no geriatric screening and was therefore excluded from part of the analyses. This led to a small sample size and could have led to selection bias and thereby hamper generalizability of results. However, to give insight in the patient selection we described their characteristics and outcomes. Since more excluded patients were treated with palliative radiotherapy, it is possible we selected an overall somewhat fitter group and thereby underestimate the prevalence of geriatric deficits. In contrast, patients that were treated with neo-adjuvant CRT plus surgery and not included in the study might have been ‘fitter’ if treating physicians thought geriatric screening was unnecessary. Third, in this observational study geriatric deficits and other patient characteristics could have influenced the choice of treatment. Therefore, confounding by indication could have occurred which hampers the comparison of treatment effects. However, the study does still provide the opportunity to study the association of GA with outcomes.
Strengths of the current study include being one of the few studies that describes all four domains of the geriatric assessment in older patients with esophageal cancer. Other studies report only limited aspects of GA. Functional status is mainly reported as ECOG PS or KPS, while previous studies have shown that GA can identify important deficits in patients judged as functionally normal by performance scores or clinicians’ judgment [Citation36,Citation37]. Moreover, this is the first study that identifies geriatric deficits as risk factor for treatment discontinuation. Finally, this study describes an older population seen in daily routine care which is representative for clinical practice.
Conclusion
All-cause mortality within one year was high, irrespective of treatment modality. Treatment discontinuation rate was high, especially in patients treated with definitive chemoradiotherapy. Geriatric assessment associates with outcomes in older patients with esophageal cancer and may inform treatment decisions and optimization in future patients, but more research is needed to establish its predictive value.
Supplemental Material
Download MS Word (12.9 KB)Supplemental Material
Download MS Word (12 KB)Supplemental Material
Download JPEG Image (70.8 KB)Acknowledgements
The authors thank Merve Varol for assistance in data collection during her internship.
Disclosure statement
The authors report no conflicts of interest.
Data availability statement
The data that support the findings of this study are available on reasonable request from the corresponding author, YvH. The data are not publicly available due to privacy concerns.
Additional information
Funding
References
- Uhlenhopp DJ, Then EO, Sunkara T, et al. Epidemiology of esophageal cancer: update in global trends, etiology and risk factors. Clin J Gastroenterol. 2020;13(6):1010–1021.
- Lowsky DJ, Olshansky SJ, Bhattacharya J, et al. Heterogeneity in healthy aging. J Gerontol A Biol Sci Med Sci. 2014;69(6):640–649.
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012; 366(22):2074–2084.
- Cloos VBM, Portier ESH, Fiocco M, et al. Neoadjuvant chemoradiotherapy followed by resection for esophageal cancer: clinical outcomes with the 'CROSS-regimen' in daily practice. Dis Esophagus. 2021; 23:doab068.
- Munch S, Pigorsch SU, Devecka M, et al. Neoadjuvant versus definitive chemoradiation in patients with squamous cell carcinoma of the esophagus. Radiat Oncol. 2019;14(1):66.
- Smit JK, Muijs CT, Burgerhof JG, et al. Survival after definitive (chemo) radiotherapy in esophageal cancer patients: a population-based study in the North-East Netherlands. Ann Surg Oncol. 2013;20(6):1985–1992.
- Stahl M, Stuschke M, Lehmann N, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol. 2005;23(10):2310–2317.
- van Ruler MA, Peters FP, Slingerland M, et al. Clinical outcomes of definitive chemoradiotherapy using carboplatin and paclitaxel in esophageal cancer. Dis Esophagus. 2017;30(4):1–9.
- Mantziari S, Teixeira Farinha H, Bouygues V, et al. Esophageal cancer in elderly patients, current treatment options and outcomes; a systematic review and pooled analysis. Cancers (Basel). 2021;13(9):2104.
- van Abbema DL, van den Akker M, Janssen-Heijnen ML, et al. Patient- and tumor-related predictors of chemotherapy intolerance in older patients with cancer: a systematic review. J Geriatr Oncol. 2019;10(1):31–41.
- van Deudekom FJ, Klop HG, Hartgrink HH, et al. Functional and cognitive impairment, social functioning, frailty and adverse health outcomes in older patients with esophageal cancer, a systematic review. J Geriatr Oncol. 2018;9(6):560–568.
- Bellera CA, Rainfray M, Mathoulin-Pelissier S, et al. Screening older cancer patients: first evaluation of the G-8 geriatric screening tool. Ann Oncol. 2012;23(8):2166–2172.
- Brooke P, Bullock R. Validation of a 6 item cognitive impairment test with a view to primary care usage. Int J Geriat Psychiatry. 1999;14(11):936–940.
- van Holstein Y, van Deudekom FJ, Trompet S, et al. Design and rationale of a routine clinical care pathway and prospective cohort study in older patients needing intensive treatment. BMC Geriatr. 2021;21(1):29.
- Guigoz Y, Lauque S, Vellas BJ. Identifying the elderly at risk for malnutrition. The mini nutritional assessment. Clin Geriatr Med. 2002;18(4):737–757.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Katz S, Ford AB, Moskowitz RW, et al. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919.
- Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–186.
- Wang X, Ge X, Wang X, et al. S-1-Based chemoradiotherapy followed by consolidation chemotherapy with S-1 in elderly patients with esophageal squamous cell carcinoma: a multicenter phase II Trial. Front Oncol. 2020;10:1499.
- Centraal Bureau voor de Statistiek (CBS). Personen in institutionele huishoudens. Statline. https://opendata.cbs.nl/statline/-/CBS/nl/dataset/82887NED/table?dl=5FF4E. 2022.
- Cooper L, Dezube AR, De Leon LE, et al. Outcomes of trimodality CROSS regimen in older adults with locally advanced esophageal cancer. Eur J Surg Oncol. 2021; 47(10):2667–2674.
- Xu C, Xi M, Moreno A, et al. Definitive chemoradiation therapy for esophageal cancer in the elderly: clinical outcomes for patients exceeding 80 years old. Int J Radiat Oncol Biol Phys. 2017;98(4):811–819.
- Mak RH, Mamon HJ, Ryan DP, et al. Toxicity and outcomes after chemoradiation for esophageal cancer in patients age 75 or older. Dis Esophagus. 2010; 23(4):316–323.
- Rahimy E, Koong A, Toesca D, et al. Outcomes and tolerability of definitive and preoperative chemoradiation in elderly patients with esophageal cancer: a retrospective institutional review. Adv Radiat Oncol. 2020;5(6):1188–1196.
- Zhao Q, Hu G, Xiao W, et al. Comparison of definitive chemoradiotherapy and radiotherapy alone in patients older than 75 years with locally advanced esophageal carcinoma: a retrospective cohort study. Medicine (Baltimore)). 2017;96(35):e7920.
- Koeter M, van Putten M, Verhoeven RHA, et al. Definitive chemoradiation or surgery in elderly patients with potentially curable esophageal cancer in The Netherlands: a nationwide population-based study on patterns of care and survival. Acta Oncol. 2018;57(9):1192–1200.
- Zhang X, Tang T, Pang L, et al. Malnutrition and overall survival in older adults with cancer: a systematic review and Meta-analysis. J Geriatr Oncol. 2019;10(6):874–883.
- Takeuchi S, Ohtsu A, Doi T, et al. A retrospective study of definitive chemoradiotherapy for elderly patients with esophageal cancer. Am J Clin Oncol. 2007;30(6):607–611.
- Tougeron D, Di Fiore F, Thureau S, et al. Safety and outcome of definitive chemoradiotherapy in elderly patients with oesophageal cancer. Br J Cancer. 2008;99(10):1586–1592.
- Jingu K, Takahashi N, Murakami Y, et al. Is concurrent chemotherapy with radiotherapy for esophageal cancer beneficial in patients aged 80 years or older? Anticancer Res. 2019;39(8):4279–4283.
- Walter F, Bockle D, Schmidt-Hegemann NS, et al. Clinical outcome of elderly patients (>/= 70 years) with esophageal cancer undergoing definitive or neoadjuvant radio(chemo)therapy: a retrospective single center analysis. Radiat Oncol. 2018; 13(1):93.
- Huang C, Huang D, Zhu Y, et al. Comparison of a concurrent fluorouracil-based regimen and a taxane-based regimen combined with radiotherapy in elderly patients with esophageal squamous cell carcinoma. Transl Oncol. 2020;13(3):100736.
- Kenis C, Baitar A, Decoster L, et al. The added value of geriatric screening and assessment for predicting overall survival in older patients with cancer. Cancer. 2018;124(18):3753–3763.
- Li D, Sun CL, Kim H, et al. Geriatric assessment-driven intervention (GAIN) on chemotherapy-related toxic effects in older adults with cancer: a randomized clinical trial. JAMA Oncol. 2021;7(11):e214158.
- Lund CM, Vistisen KK, Olsen AP, et al. The effect of geriatric intervention in frail older patients receiving chemotherapy for colorectal cancer: a randomised trial (GERICO). Br J Cancer. 2021;124(12):1949–1958.
- Jolly TA, Deal AM, Nyrop KA, et al. Geriatric assessment-identified deficits in older cancer patients with normal performance status. Oncologist. 2015;20(4):379–385.
- Wedding U, Kodding D, Pientka L, et al. Physicians' judgement and comprehensive geriatric assessment (CGA) select different patients as fit for chemotherapy. Crit Rev Oncol Hematol. 2007;64(1):1–9.
