 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
to find clinical features that can predict prognosis in patients with oligometastatic disease treated with stereotactic body radiotherapy (SBRT)
Material and methods
Patients with less than 5 metastases in less than 3 different body sites were included in the analysis. Various clinical and treatment parameters were analyzed to create a Cox proportional hazard model for Overall Survival (OS). Subsequently, significant variables were used to create a score
Results
997 patients were analyzed. Median OS was 2.61 years, 1 and 3 years OS was respectively 85% and 43%. Location of the primary tumor, performance status, site of irradiated metastases, presence of extratarget non irradiated lesions and RT dose were significant prognostic factors for OS. These parameters were used to create a score and to distinguish three different classes, with median OS of 5.67 years in low risk, 2.47 years in intermediate risk and 1.82 years in high risk group.
Conclusion
moving from easily accessible clinical parameters, a score was created to help the physician’s decision about the better treatment or combination of treatments for the individual patient.
Background
The concept of an intermediate state between localized tumor and widespread metastatic disease was formally described in 1995 [Citation1], although sporadic case reports of metastatic patients treated with curative intent can be found in literature since 1930s [Citation2–4].
From the first definition by Drs. Hellman and Weichselbaum, there has been a constant increase of the interest toward local ablative approaches in such patients, with thousands of patients treated and hundreds of published papers. More recently, published prospective evidence (small phase II randomized trials) suggested the efficacy of combining local and systemic treatments to improve the prognosis of patients with oligometastatic disease [Citation5–8].
However, despite the great amount of available data, an exact definition of ‘oligometastases’ is still missing, and patients are currently identified with a limited number of metastases (usually one to five) in few organs (one to three), theorizing an indolent biology still undefined. Recently, European Society for Radiotherapy and Oncology (ESTRO) and European Organization for Research and Treatment of Cancer (EORTC) published a consensus recommendation for characteristics and classification of oligometastases still partially linked to number of metastases as defining parameter [Citation9]. The same societies also launched a prospective trial, the OligoCare study, to collect a great amount of data, that in the future could be useful for a more precise and reliable definition.
It is more and more evident that current definition is too limited and that what we now call ‘patients with oligometastatic disease’ are indeed a very heterogeneous population. It is also clear that only a percentage of them can take benefit from an aggressive local approach, at least for an overall survival endpoint.
Attempts to move beyond the number of metastases are still few. The most promising data are those that aimed at identifying genomic signature to distinguish the prognosis of apparently similar patients with oligometastatic disease. A study from the University of Chicago analyzed specific microRNAs from patients treated with lung resection [Citation10] or stereotactic body radiation therapy (SBRT) [Citation11] for oligometastatic disease, differentiating oligometastatic vs. polymetastatic phenotypes linked to the clinical course of the patients. Similar approaches have been attempted by other authors [Citation12–13], but the results of these studies are still missing validation in larger studies and are quite far from clinical implementation on a larger scale.
Waiting for prospective data, the more actual way, although less precise, is the evaluation of clinical and treatment parameters that are easily accessible for all patients to create prognostic models and possibly classify patients according to the predicted survival. Some experiences have already been published although on a limited number of patients [Citation14–16].
With the present work we aim to analyze different possible prognostic features for overall survival in about 1000 patients with oligometastatic disease treated with SBRT in our institution and to generate a score that can be used as supporting tool at the first clinical examination in a radiation oncology department.
Material and methods
This is a retrospective analysis on patients with oligometastatic disease treated between 2014 and 2018 with SBRT in a single institution. Patients were included if they were affected by less than five lesions in no more than three different organs. All histologies were allowed apart from small cell lung cancer and hematologic malignancies. The local ethical committee acknowledged and allowed this retrospective study. The study was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). All patients provided full written informed consent. The data that support the findings of this study are available from the corresponding author, [DF], upon reasonable request.
We included patients with synchronous or metachronous lesions; previous or concomitant systemic therapies (chemotherapy, endocrine therapy, immunotherapy or target therapy) were allowed. A lesion was defined synchronous if diagnosed within 1 month from the primary diagnosis. Patients were categorized as follows:
Oligometastatic de novo if they did not receive any previous systemic therapy for the stage IV diagnosis
Induced oligometastatic if they were rendered ‘oligo’ by the administration of systemic therapies with a partial response (induced oligorecurrence or oligopersistence according to the most recent EORTC definition [Citation9])
Oligoprogressive if they were affected by more metastases, but with only few of them progressing during systemic therapy (induced oligoprogression according to the most recent EORTC definition)
In case of oligoprogression, only progressing metastases were irradiated, while the remnant lesions (stable or responding to systemic therapy) were simply monitored. At least 6 months stability of the other metastases was required.
Adjuvant therapies were recorded if they were received up to 6 months after SBRT.
All patients were treated with SBRT with radical intent, dose and fractionation schedules were chosen according to metastatic sites, previous treatment and proximity of organs at risk.
Biologically equivalent dose (BED) was calculated according to the following formula:
where N is the number of treatment fractions, d is the dose per fraction in gray, and a/b is the dose at which the linear and quadratic components of cell kill are equal. In case different metastases were treated with different doses in the same patient, the lowest BED per patient was used for this analysis.
All patients were staged, as minimum requirement, with brain, thorax and abdomen CT and PET FDG or PET choline (if affected by prostate cancer). Brain MRI was performed if possible, in case of brain metastases.
After treatment patients were followed up according to the international guidelines applicable for their primary histology.
Statistical analysis
The analysis was performed following the framework of Van den Begin et al. [Citation15] and employing the R package survival [Citation17]. Overall survival was calculated from the last day of SBRT until last follow up visit or death from any cause.
A Cox proportional hazard model was fitted to the data, adjusting for covariates as in : Gender, Location of the primary tumor, Histology of the primary tumor, Treatment of the primary tumor, Presence of comorbidities, Performance status, Timing of metastases, Oligometastatic type, Previous local ablative treatments, Previous chemotherapy lines, Age at start of SBRT, Number of irradiated lesions, Location of irradiated lesions, Extra target and Biologically equivalent dose (BED).
Table 1. Patient characteristics.
Only variables corresponding to a significance level lower than 1% were retained. PH assumption was verified through log-negative-log KM and tests on Shoenfeld residuals, as implemented in the survival R package routines.
Subsequently, a score for patients with oligometastatic disease was set up. For each patient, the score is computed starting from the significant (p-val < 0.01) covariates retained in the multivariate Cox predictive model, whose hazard function is
being x1 = Location of the primary tumor (levels = colorectal, lung, pancreas, prostate); x2 = Performance status (levels = 0,1,2); x3 = Location of irradiated lesions (levels = Lung, Liver); x4 = Extra target (levels = yes, no); x5 = BED.
Then, the coefficients of the estimated model *1000 are retained, and then multiplied for the values of the corresponding covariates in each patient. Scores are then obtained standardizing these values (i.e., subtracting the minimum, then dividing for the range), and multiplying by 100, in line with what is reported in Van den Begin et al. [Citation15].
At https://fraiev.shinyapps.io/Oligoscore_webapp/, an interactive web application shows the implemented method and allows for predictions for a new patient for whom suitable inputs are provided.
Results
After institutional database review, 997 patients were included in the analysis.
The majority was males (555 patients, 55.7%); most common primary tumors were colorectal cancer (278 patients, 27.9%) and lung cancer (246 patients, 24.7%). Median age at cancer diagnosis was 65 ± 13 years. Main patients’ characteristics are listed in . All patients received a form of treatment or combination of treatments for their primary tumor, details are available in .
About one third of patients were treated for isolated lung metastases (336 patients, 33.7%), other common sites were lymph-nodes (215 patients, 21.6%), liver (192 patients, 19.3%) and brain (133 patients, 13.3%). Thirty-two patients (32, 3.2%) were irradiated for isolated adrenal gland metastases; the remaining part of cases was irradiated on more than one organ at the same time. No patient was treated on more than 2 organs.
The majority of patients was treated for a single metastasis (583, 58.5%) or two metastases at the same time (268, 26.9%). Only few patients (40, 4%) were irradiated on 4 or 5 metastases simultaneously. In the oligoprogressive patients, 77 (46.7%) of them had more than five metastases in the body, although less than five were progressing and were therefore irradiated.
Median BED was 105 Gy, 540 patients (54.2%) were treated with a BED > 100 Gy. In Supplementary Table 1, different dose and fractionation schedules prescribed according to metastatic site are reported.
A local relapse of the irradiated lesion during follow up occurred in 182 patients (18.3%). The highest local recurrence rate was recorded for adrenal gland metastases (24%, 12 patients), followed by brain (21.7%, 30 patients), liver (17.6%, 43 patients), lung (17%, 67 patients) and nodes (12.3% 32 patients. Median local control (for patients experiencing local progression) was 10.5 months (range 1.3–73.1 months), LC at 1 and 3 years was 88% and 73%.
Distant progression occurred in the majority of patients (714; 71.6%), in most cases patients progressed again in an ‘oligo’ way with less than 5 new metastases (520 cases; 52.2%), still amenable to further local therapies. Median PFS was 9 months, 1 and 3 years PFS was 38% and 15%.
After a median follow up of 2.52 years (range 1.9–73.1 months), 149 patients (14.9%) were alive with no evidence of residual disease, 48 patients (4.8%) were alive with only local disease (already irradiated although persisting), 345 patients (34.6%) were alive with further metastases, 419 patients (42%) died for disease progression and 36 patients (3.6%) died for other causes not related to oncologic disease.
Median OS was 2.61 years (range 1.9–73.1 months), 1 and 3 years OS was respectively 85% and 43%.
As shown in , the score identifies eight elements, which can be considered either as risk factors or protective factors for OS. Considering e.g., the Location of the primary tumor (whose baseline level is Colorectal) Lung and Pancreas appear to increase the risk, while Prostate does the opposite. At the same time, both levels of Performance status greater than the baseline level 0 increase risk significantly. Concerning site of irradiated metastases, liver lesions appear to have a negative prognostic value. Also the presence of extratarget non irradiated lesions confers an increasing risk of death. On the contrary, BED as a continuous variable has a mild protective significance.
Table 2. Score associated with statistically significant variables for Overall Survival (OS).
As an example, consider patient A with the following characteristics:
Location of the p.t.: Colorectal;
Performance status: 0;
Location of irradiated lesions: Lung;
Extra target: no;
BED: 105.6.
Summing the contribution of every matching categorical covariate and the value of BED multiplied for its coefficient, the resulting value is −316.8, which, rescaled on the [0, 100] interval, corresponds to 51.4. Since the tertiles defining the risk classes are 59.0 and 66.6, patient A falls in the low risk category.
For further clarification, consider a patient B which features instead:
Location of the p.t.: Colorectal;
Performance status: 1;
Location of irradiated lesions: Liver;
Extra target: no;
BED: 129.15.
This individual would have a raw score of 536.5 and a normalized one of 71, thus belonging to the high risk class.
shows the Kaplan Meier curves of the three risk classes, along with a table that illustrates the corresponding impact of censoring. The three classes appear to be well separated, except for minor overlaps after the 4-year mark, due to the low number of at-risk individuals at that time point. Summaries for the three risk classes are reported in .
Figure 1. Kaplan Meier estimator divided by risk class. Class 1 represents low risk, Class 2 medium risk and Class 3 high risk.
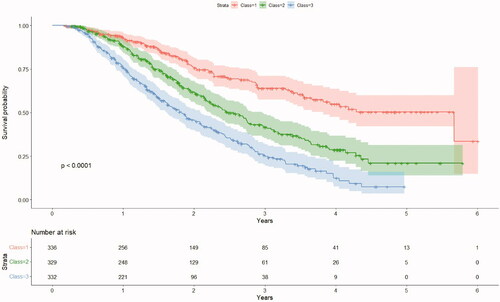
Table 3. Class summaries.
Discussion
In this paper we present a practical approach for radiation oncologists to classify patients with oligometastatic disease according to easily accessible clinical features. By analyzing data from almost 1000 patients treated in our institution with SBRT for oligometastases, we were able to categorize patients in three groups with statistically different OS (5.67 years in low risk, 2.47 years in intermediate risk and 1.82 years in high risk group) p-val < 0.0001.
Such different prognosis could translate in a different approach from the therapeutic point of view. Indeed, while in low risk group of patients local treatment by itself or along with not modified systemic therapy could be considered enough; in the high risk group there is probably the need for treatment intensification to improve the prognosis.
The present study includes essentially patients with one or two metastases, predominantly from colorectal and lung cancer. Patients with bone metastases were not included in this analysis, since in most cases they were not treated with radical doses or SBRT and the few treated with SBRT were such a small number that it was not possible to identify a specific category for them. We therefore focused on visceral metastases, including nodal lesions.
While there is still little space to increase the availability and efficacy of local approaches, particularly for what concerns SBRT that is already proven as a safe and effective treatment, on the contrary there is still a lot to do to improve the selection of patients. Our study is similar to previously published experiences although with a higher number of patients. The experience published by Hong et al. allowed the identification of five different prognostic classes simply moving from easily accessible clinical parameters, like histology, age, number of metastases and disease free interval. More similarly to our experience, Van der Begin et al. created the METABANK score [Citation15], analyzing clinical features of 403 patients treated with SBRT for 760 metastases. Authors found 5 independent prognostic factors (male sex, synchronous timing of oligometastases, brain metastasis, non-adenocarcinoma histology, KPS <80), which allowed them to discriminate 4 different classes with median survival going from 7.9 to 41.2 months. With a similar work, although on almost 1000 patients, we identified 8 prognostic factors, three of them related to the primary histology, one to the site of the irradiate lesion (liver in our case instead of brain), two correlated with performance status like for Van der Begin et al. We also found a strong negative prognosis for patients with locally untreated although controlled metastatic disease (essentially the oligoprogressive and some oligo-induced patients) like in our previously published study [Citation17] and a mild continuous impact of BED on prognosis. These two latter factors suggest in our opinion the need for a complete ablation of the macroscopic disease with radical doses whenever possible.
In our result, sex, timing, intracranial disease did not influence OS differently from the METABANK results. The heterogeneity of both series is probably the major limitation and an obstacle to the extrapolation of similar results in clinical practice. Single tumor predictive scores should probably be more consistent and reproducible.
It is becoming more and more evident that oligoprogression is a complete distinct scenario. Moreover, considering the increasing use of target therapies and immunotherapies, it is likely that oligoprogression will become even more common. Ablating all persisting disease and not only resistant metastases, as done in our study, could be more advantageous and should be matter of specific studies in our opinion.
We believe that clinical parameters are just the tip of the iceberg in oligometastatic setting. The advantage is that they are easily accessible and reproducible; however, they cannot represent the right way for identifying these patients. There is a need for studies that identify the underlying biology of the disease, through the analysis of genetic and epigenetic factors, without forgetting the possible role of the immune system also in this clinical scenario.
For instance, a genetic signature predicting metastatic cascade has been found in patients affected by pancreatic cancer with the autopsy series by Iacobuzio-Donahue et al. [Citation18]. They found that SMAD4 loss predicts a metastatic evolution and this could stimulate further clinical research to investigate its role in patients with oligometastatic pancreatic cancer.
In the world of epigenetic signatures, there is increasing literature focused on the role of microRNAs. For instance, Lussier et al. in a small series of oligometastatic lung cancer patients found that overexpression of the miR-200 family was correlated with polymetastatic progression after treatment [Citation10]. These are patients, that, although clinically oligometastatic, do not benefit in terms of survival from local approaches, since the biology of their disease is aggressive more than indolent.
Pitroda et al. [Citation13] created a score for hepatic colorectal metastases combining clinical parameters and microRNAs expression. This model performed better than the clinical model by itself but also better than the epigenetic model by itself, underlying that clinical features cannot be completely neglected.
The present study has some intrinsic limitations related to its retrospective nature, to the heterogeneity of the series and to the lack of external validation. However, the high number of enrolled patients should mitigate these biases. Our project will go on with the creation of a similar score for some of the commonest histologies, like non small cell lung cancer (NSCLC) and colorectal cancer, adding to the model some parameters that are known prognostic or predictive factors for that single tumor (like for instance mutational status in NSCLC). At the same time, we also plan to connect this clinical data on single histologies with genetic analysis and radiomic features to create a more precise signature. Finally, in a survival context, validation is not a straightforward task to accomplish, even in case of high performances on the training test. A future direction for the work in this sense might be the application of the score to the risk stratification of an external database for which the association between prognosis and risk has already been verified by clinicians.
Conclusion
We present an easily accessible prognostic score for patients with oligometastatic disease candidate to SBRT ablation. Three different prognostic classes can be identified moving from eight parameters. This score could help the physician’s decision about the better treatment or combination of treatments for the individual patient.
Supplemental Material
Download MS Word (53.5 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Hellman S, Weichselbaum R. Oligometastases. J Clin Oncol. 1995;13(1):8–10.
- Barney J, Churchill E. Adenocarcinoma of the kidney with metastasis to the lung cured by nephrectomy and lobectomy. J Urol. 1939;42(3):269–276.
- Rosenberg SA. Surgical treatment of metastatic cancer. Philadelphia (PA): Lippincott Williams & Wilkins; 1987.
- Peters LJ, Milas L, Fletcher GH. The role of radiation therapy in the curative treatment of metastatic disease. Symp Fundam Cancer Res. 1983;36:411–420.
- Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (sabr-comet): a randomised, phase 2, open-label trial. Lancet. 2019;393(10185):2051–2058.
- Gomez DR, Tang C, Zhang J, et al. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: long-term results of a multi-institutional, phase II, randomized study. J Clin Oncol. 2019;37(18):1558–1565.
- Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36(5):446–453.
- Iyengar P, Wardak Z, Gerber DE, et al. Consolidative radiotherapy for limited metastatic non-small-cell lung cancer: a phase 2 randomized clinical trial. JAMA Oncol. 2018;4(1):e173501.
- Guckenberger M, Lievens Y, Bouma AB, et al. Characterisation and classification of oligometastatic disease: a European Society for radiotherapy and oncology and European Organisation for research and treatment of cancer consensus recommendation. Lancet Oncol. 2020;21(1):e18–e28.
- Lussier YA, Khodarev NN, Regan K, et al. Oligo- and polymetastatic progression in lung metastasis(es) patients is associated with specific microRNAs. PLoS One. 2012;7(12):e50141.
- Lussier YA, Xing HR, Salama JK, et al. MicroRNAs expression characterizes oligometastasis(es). PLoS One. 2011;6(12):e28650.
- Wong AC, Watson SP, Pitroda SP, et al. Clinical and molecular markers of long-term survival after oligometastasis-directed stereotactic body radiotherapy (SBRT). Cancer. 2016;122(14):2242–2250.
- Pitroda SP, Khodarev NN, Huang L, et al. Integrated molecular subtyping defines a curable oligometastatic state in colorectal liver metastasis. Nat Commun. 2018;9(1):1793.
- Hong JC, Ayala-Peacock DN, Lee J, et al. Classification for long-term survival in oligometastatic patients treated with ablative radiotherapy: a multi-institutional pooled analysis. PLoS One. 2018;13(4):e0195149.
- Van den Begin R, Benedikt Engels B, Christine Collen C, et al. The METABANK score: a clinical tool to predict survival after stereotactic radiotherapy for oligometastatic disease. Radiother Oncol. 2019;133:113–119.
- Franceschini D, De Rose F, Franzese C, et al. Predictive factors for response and survival in a cohort of oligometastatic patients treated with stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2019;104(1):111–121.
- Therneau TM. A package for survival analysis in S. version 2.38. 2015. https://CRAN.R-project.org/package=survival) of the R software (R Core Team 2020). R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing. https://www.R-project.org/
- Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27(11):1806–1813.
