Abstract
Background
Identifying pretreatment blood markers that distinguish prognostic groups of patients with advanced pancreatic ductal adenocarcinoma (PDAC) under first-line FOLFIRINOX chemotherapy has the potential to improve management of this condition. Aim of this study was to determine the prognostic utility of a range of pretreatment, inflammation-related, blood cell markers in this group of patients.
Material and methods
Data from a training cohort were analyzed to identify potential pretreatment blood markers correlating to survival outcomes. The most informative markers were further analyzed in a validation cohort comprised patients from a geographically separate cancer center undergoing the same treatment.
Results
A total of 138 consecutive patients receiving FOLFIRINOX chemotherapy between 2010 and 2019, constituted the training cohort. Neutrophil/lymphocyte (NLR), monocyte/lymphocyte (MLR), and platelet/lymphocyte ratio (PLR) as well as the systemic inflammatory response index (SIRI) and CA19.9 showed prognostic significance in addition to tumor stage. A pretreatment SIRI score cutoff of 2.35 differentiated between a poor prognostic group with median overall survival (mOS) 5.1 months and a better prognostic group, mOS 12.5 months. SIRI ≤/> 2.35 was predictive of mOS in patients with locally advanced and metastatic PDAC. SIRI was confirmed as a prognostic marker in a validation cohort of 67 patients with mOS of 13.4 months and 6.3 months for those with SIRI ≤ 2.35 and >2.35, respectively. Additional analysis revealed baseline SIRI as being prognostic within additional subgroups of patients in both cohorts.
Conclusions
This large, retrospective, analysis of real-world patients receiving first-line FOLFIRINOX chemotherapy for advanced PDAC has identified the pretreatment blood SIRI as a strong prognostic marker for survival. This will allow better counseling of patients with regards to the benefits of treatment, improved stratification within clinical trials, and potentially identify groups of patients for novel therapy trials as first-line treatment.
Introduction
Pancreatic ductal adenocarcinoma (PDAC), with an incidence of approximately 10−20/100,000 population, is the fourth leading cause of cancer-related death in developed countries [Citation1] and is expected to become the second most common cause of cancer death by 2030 [Citation2]. The majority of patients present with inoperable locally advanced or metastatic disease, with the result that treatment aims is directed toward symptom relief, disease control, and potential prolongation of survival rather than cure [Citation3]. However, existing treatment options have extended survival by only a few months with median life expectancy in the advanced setting remaining less than 12 months and 5-year survival, for metastatic disease, being only 2% [Citation4,Citation5].
The combination of 5-fluorouracil (5-FU), leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX regime) has shown the highest response and survival rates of any systemic treatment in the first-line setting in advanced PDAC, as observed in the randomized phase III study by Conroy et al. [Citation4]. However, as median progression-free survival is generally short lived, around 6 months, there are a group of patients that do not benefit, as seen in the FOLFIRINOX study where 15% of participants showed disease progression as their best response. Identification of molecular and clinical biomarkers remains an unmet need in attempting to determine likely benefit of treatment in specific groups of patients [Citation6]. Markers of tumor inflammation, especially neutrophil-to-lymphocyte ratio (NLR) or platelet-to-lymphocyte ratio (PLR), have been used in prognostication and can be used as clinical biomarkers in patients with solid tumors including breast, renal, prostate, and gastro-oesophageal cancer [Citation7,Citation8]. Similar markers have also been evaluated in PDAC [Citation9–11] but limited evidence exists in patients who received FOLFIRINOX as first-line chemotherapy for advanced disease [Citation12–14]. In addition, most of these markers have been tested in small cohorts without further validation.
In this study, a panel of inflammatory markers has been explored as a potential prognostic indicator for patients with locally advanced and metastatic PDAC treated 1st-line with FOLFIRINOX, and these data have been further validated in a separate tertiary center cohort.
Methods
Population and patient data collection
Patients selected for inclusion in this study were those with inoperable locally advanced or metastatic PDAC, for whom palliative FOLFIRINOX chemotherapy was the initial systemic treatment. An initial training cohort consisted of all patients treated with FOLFIRINOX between 2010 and 2019 at Leeds Teaching Hospitals and Mid-Yorkshire Hospitals NHS Trusts, England. A validation cohort was selected from a database of patients with PDAC similarly treated at The Christie NHS Foundation Trust, Manchester, England. The size of this cohort was calculated statistically, as described below, according to the results of the training cohort. Inclusion criteria included histological or cytological confirmation of pancreatic adenocarcinoma (including those with acinar and squamous differentiation but excluding neuroendocrine, small cell or other variants), unresectable locally advanced or metastatic stage, performance status 0–2 by Eastern Cooperative Oncology Group (ECOG PS), first-line treatment with at least one cycle of FOLFIRINOX and adequate follow-up information. In those patients with locally advanced disease at the end of chemotherapy, and after multidisciplinary team (MDT) review, consolidation radiotherapy was offered. Patients with active infection or taking corticosteroids before starting the first cycle of chemotherapy were excluded from the study.
Approval for collection and use of anonymised and aggregated patient data was obtained from individual site audit committees (The Christie NHS Foundation Trust audit committee approval: reference 16/1812; Leeds Teaching Hospitals NHS Trust: audit number 9680; Mid-Yorkshire Hospitals NHS Trust: audit number 1363).
Demographic, disease and treatment characteristics, best response to chemotherapy, and survival status were collected from electronic patient case records (EPRs) of the respective treating hospitals. All patients were initiated on a FOLFIRINOX regimen consisting of: oxaliplatin 85 mg/m2, irinotecan 150 mg/m2, calcium folinate 350 mg, all by intravenous (IV) infusion; bolus 5-FU 400 mg/m2, 46 h continuous infusion 5-FU 2400 mg/m2. All drugs were given/commenced on day 1 and the regimen repeated every 14 d. Treatment delays and modifications were introduced according to the original phase III trial protocol and growth factors were administered as per institutional policy. On treatment radiological assessment was performed approximately 12 weekly, with response assessed using RECIST 1.1 criteria by the treating physicians. Post-treatment assessment was not protocolled with disease progression being detected clinically, biochemically, or radiologically as appropriate. No central radiological confirmation of response was performed.
Hematological biomarkers
The baseline blood levels of white blood cells, neutrophils (NEUT), monocytes (MONO), lymphocytes (LYMPH), and platelets (PLT), as well as serum albumin (ALBUM) were collected from the EPR databases of the treating hospitals. All tests were performed within 48 h prior to starting the first cycle of FOLFIRINOX chemotherapy. Blood biomarker levels were calculated as follows: NLR (= NEUT divided by LYMPH levels), monocyte-to-lymphocyte ratio (MLR = MONO divided by LYMPH levels), systemic inflammatory response index (SIRI = NEUT multiplied by MONO and divided by LYMPH levels), PLR (= PLT divided by LYMPH levels) and prognostic nutritional index [PNI = serum ALBUM concentration (g/L) plus [0.005 multiplied by lymphocyte count (number/mm3)] [Citation15,Citation16].
Statistical methods
The statistical analyses were performed using Statistical Package for the Social Sciences for Windows version 22 (SPSS, Inc., Chicago, IL, USA) and MedCalc. Categorical characteristics were compared by chi-square and continuous variables by Mann–Whitney non-parametric test. Baseline characteristics included age, gender, ECOG PS, stage (8th edition TNM, UICC/AJCC), primary tumor site, baseline serum CA19-9 levels, and history of prior surgery or chemotherapy.
The primary objective of the study was to identify the optimal cutoff best predicting 1-year overall survival (OS) for each of the baseline blood biomarkers explored. OS was defined as the time from day 1 of FOLFIRINOX chemotherapy until death or last confirmed patient contact. At the time of analysis, all alive patients had a minimum of 12 months of follow-up. Secondary objectives included comparison of radiological response (stable disease, partial, or complete response or progression) to treatment between patients with biomarker level above vs. below the predefined cutoff.
The training cohort was used to identify this biomarker and cutoff. The prognostic impact of baseline blood biomarker levels, adjusted for baseline characteristics, was tested by multivariable Cox proportional hazard models, with a forward selection procedure and a selection criterion of p < .10. In univariate and multivariable analysis, the natural logarithm (ln) of continuous variables (CA19-9, NLR, MLR, SIRI, PLR, and PNI) was examined to improve the interpretation of HRs. Receiver operation characteristics (ROC) curves were used to assess the sensitivity and specificity of the best prognostic biomarker in the training cohort. Then the Youden index was calculated for each biomarker level as Youden index = sensitivity + specificity − 1 [Citation17]. The baseline blood biomarker level with the highest Youden index was selected as the best cutoff.
The prognostic significance of the selected cutoff level was assessed in the validation cohort, whose size was determined according to the 1-year OS associated with the biomarker cutoff in the training cohort. The selected biomarker cutoff level was considered as prognostic if the difference of 1-year OS in the patients with biomarker levels below and above this cutoff reached the predefined difference set at the training cohort. OS curves, according to the selected biomarker cutoff levels, were constructed by the Kaplan–Meier method and median OS was compared by log-rank test for each biomarker separately. All comparisons were two-tailed with level of significance p < .05.
Results
Training cohort characteristics and prognostic factors
The training cohort included 138 patients with locally advanced or metastatic PDAC who received first-line FOLFIRINOX. Basic patient and disease characteristics are shown in . Baseline blood biomarker levels are described in . Prognostic factors in the training cohorts are shown in Table S1. Tumor stage as well as blood parameters, such as NLR, MLR, SIRI, PLR, and serum CA19-9 demonstrated independent prognostic significance.
Table 1. Basic patient and disease characteristics and blood parameters levels in the training and validation cohorts.
Best prognostic biomarker cutoff
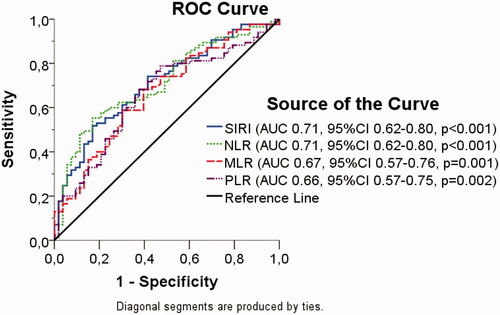
After a median follow-up of 42.7 months (range, 0.3–64.9), only 10 patients were alive in the training cohort. All alive patients had a minimum follow-up of 12 months. Therefore, 1-year survival rate was used as the endpoint to determine the best prognostic biomarker cutoffs in the training cohort. In view of their significance, on multivariate analysis, NLR, MLR, SIRI, and PLR were selected as baseline blood biomarkers of interest. A receiver operator characteristics (ROC) curve was used to calculate the sensitivity and specificity that each SIRI, NLR, MLR, and PLR level predict 1-year OS (). Area under the ROC curve (AUC) for SIRI, NLR, MLR, and PLR were calculated (). The best AUC values were achieved for NLR and SIRI. The SIRI was selected because it combines NEUT, MONO, and LYMPH levels. The maximum Youden index was used to identify the best prognostic SIRI cutoff level. As shown in Figure S1, the highest Youden index level was 2.35 indicating it as the best prognostic SIRI cutoff.
Estimation of the validation cohort sample size
In the training cohort, 1-year OS of patients with SIRI > 2.35 was 17.0 vs. 51.7% with SIRI ≤ 2.35. The sample size of the validation cohort was 67 patients, considering a known 1-year OS of 17.0% and an expected 1-year OS of 52% with a superiority margin of 20%, alpha = 5% and beta = 20%. Sixty-seven patients were randomly included from the Christie NHS Foundation Trust database.
Validation cohort characteristics and prognostic factors
The patient and disease characteristics of the validation cohort are depicted in . Patients of the validation cohort were, on average, younger and had worse ECOG PS than the training cohort but with no significant differences in tumor characteristics. The blood count levels are described in . Patients of the training cohort had slightly but statistically higher platelet and lower ALBUM levels. Also, MLR, SIRI, and PLR were statistically higher in the training cohort, while PNI was higher in the validation cohort. Cox proportional hazard models showed that only MLR and SIRI were confirmed independent prognostic factors for OS in the validation cohort (Table S1).
Confirmation of proposed SIRI cutoff level in the validation cohort
In the validation cohort, 1-year OS of patients with SIRI > 2.35 was 29.4 vs. 62.0% with SIRI ≤ 2.35. Thus, the difference between 1-year OS was 32.6%, which exceeded the 20% superiority margin of the statistical hypothesis and was remarkably close to the difference of 34.7% that was achieved in the training cohort. Therefore, SIRI = 2.35 was confirmed as a valuable prognostic biomarker cutoff level.
A baseline blood SIRI cutoff = 2.35 was also prognostic in both the training cohort with regard to median OS (SIRI ≤ 2.35 vs. SIRI > 2.35, median OS 12.5 vs. 5.1 months, respectively, p < .001) (), and the validation cohorts (SIRI ≤ 2.35 vs. SIRI > 2.35, median OS 13.4 vs. 6.3 months, respectively, p = .010) (). Subgroup analysis in the total population including both cohorts, showed that SIRI was prognostic in the group of patients with locally advanced disease (mOS 13.7 vs. 8.6, p < .001) (Figure S2(a)), as well as in those with metastatic disease (mOS 11.9 vs. 4.7 months, p < .001) (Figure S2(b)). SIRI, with a cutoff level of 2.35, had independent prognostic significance in both the training and the validation cohorts ().
Figure 2. Prognostic significance of SIRI > 2.35 vs. ≤ 2.35 in the training (a) and validation (b) cohort.
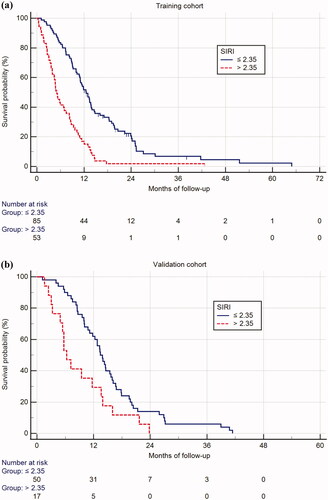
Table 2. Multivariate analysis of prognostic factors for overall survival in the training and the validation cohort including SIRI cutoff = 2.35.
Association of SIRI with disease response to treatment in the entire population
In the entire population of 205 patients (training + validation cohorts), 57 (27.8%) patients demonstrated a radiological response (R) to FOLFIRINOX (2 had complete response and 55 had partial response), 68 (33.2%) had stable disease (SD), 49 (23.9%) had progressive disease (PD), while 31 (15.1%) were not assessed radiologically because they experienced early clinical deterioration (ECD). Median OS was 13.9 months (95% CI 13.4–14.4) for patients with R, 13.7 months (95% CI, 11.0–16.4) for SD, 5.7 months (95% CI 4.4–7.0) for PD, and 2.6 months (95% CI 1.1–4.2) for ECD. Median OS of patients with R and SD was similar (p = .788) and was significantly longer than median OS of patients with PD (p < .001 for both comparisons) and ECD (p < .001 for both comparisons). The difference between median OS of patients with PD and ECD was weaker (p = .026). Patients were allocated into two groups, the first including R and SD (disease control) and the second PD and ECD (disease progression). Median OS of patients with disease control was 13.8 months (95% CI 12.9–14.8) and 4.9 months (95% CI 4.1–5.7) for disease progression (p < .001).
Response to treatment was associated with SIRI levels (Table S2 Chi-square p < .001). SIRI levels > 2.35 were associated with a lower chance of achieving disease control (R + SD) compared to levels ≤ 2.35 (41.4 vs. 71.1%, p < .001). Median SIRI levels of patients with disease progression were 2.43 (range, 0.5–21.75, SD 3.44) vs. 1.29 (range, 0.31–13.96, SD 2.29) with disease control (Mann–Whitney, p < .001). Subgroup survival analysis showed that SIRI cutoff = 2.35 was prognostic for both the groups of patients who achieved disease control and experienced disease progression with chemotherapy (Figure S3). Among patients who achieved disease control, SIRI > 2.35 was associated with significantly shorter median OS compared to SIRI ≤ 2.35 (10.5 vs. 15.4 months, respectively, p = .002). Similarly, in patients who experienced disease progression, SIRI > 2.35 was associated with significantly shorter median OS compared to SIRI ≤ 2.35 (3.6 vs. 8.4 months, respectively, p < .001).
Discussion
Advanced pancreatic cancer remains a challenging clinical condition to treat. Cytotoxic chemotherapy continues as the main treatment option, and although use of FOLFIRINOX provides the best survival figures, prognosis remains dismal. The identification of clinically validated prognostic and predictive markers could allow individual treatment decisions to be based on the likelihood of benefit. Through increased understanding of genetic and transcriptomic alterations in PDAC certain molecular signatures have been suggested as predictors of treatment response and OS [Citation6]. However, their use in clinical practice may be limited through the availability of resources to process tumor specimens sufficiently rapidly to influence management decisions.
Clinical biomarkers, on the other hand, have been used extensively across multiple tumor types to identify prognostic and predictive groups [Citation18]. In our study, a number of inflammatory biomarkers, derived from routinely measured blood indices, were assessed.
In the training cohort increasing NLR, MLR, SIRI, PLR, and CA19.9 were related with worse outcome in concordance with previous studies [Citation9]. This was confirmed for NLR, MLR, and SIRI in the validation cohort. When both cohorts were combined, only SIRI levels remained as an independent prognostic biomarker in terms of OS. As expected those patients responding to treatment had reduced risk of death, as well as patients receiving consolidation radiotherapy.
Increasing SIRI values have been shown to predict worse survival outcomes in a variety of tumor types [Citation19]. SIRI has been shown to have prognostic value in patients with pancreatic cancer undergoing curative surgery, chemo-radiotherapy, or gemcitabine-based palliative chemotherapy [Citation14,Citation15,Citation18,Citation20]. Our study confirms and validates the prognostic value of SIRI in the largest cohort of patients with locally advanced and metastatic pancreatic adenocarcinoma treated with FOLFIRINOX reported to date. Pacheco-Barcia et al. also identified SIRI (using a cutoff of 2.3) as potentially prognostic of OS in a group of patients with pancreatic cancer treated with various chemotherapy regimens. However, only 22 patients treated with a modified FOLFIRINOX regimen were included in the study.
One of the strengths of this study is the large number of patients treated in three different hospitals with a standard FOLFIRINOX regimen. The patient group is thus representative of the standard clinical population of patients with advanced PDAC and survival data is broadly similar to the original FOFIRINOX phase III study [Citation4] and previous real-world reported outcomes [Citation21,Citation22]. It is notable that 15% of all patients commencing chemotherapy stopped treatment prior to a first planned radiological assessment, in most cases, through rapid clinical deterioration. This reflects the underlying nature of advanced pancreatic cancer but also emphasizes the need to select patients better prior to treatment initiation. Of interest our results also show that a SIRI < 2.35 is associated with a greater chance of radiological response to treatment, compared to patients with SIRI > 2.35. In addition, it can differentiate patients into better and worse surviving cohorts within those showing radiological response.
Different SIRI cutoff levels have been used in the literature, varying between 0.69 and 2.3 in pancreatic cancer [Citation14,Citation15,Citation20,Citation23]. Although there is no standard cutoff, a value around 2 seems to be more often reported in the literature. The level of 2.35 in our study is in agreement with a recent study [Citation14] which also assesses the role of SIRI in patients with advanced PDAC.
Despite the strengths of our study, we acknowledge the retrospective nature of the presented data. Patients in the training set were all patients receiving FOLFIRINOX chemotherapy in two hospitals during the time period of interest. We feel, therefore, that this represents real-world data reflecting patients seen in everyday clinical practice. In addition, we have not analyzed whether the number of cycles of chemotherapy received by each patient or chemotherapy toxicities influencing duration of treatment were different between patients above or below the SIRI cutoff. However, we have seen nothing to suggest an imbalance that could significantly skew the results.
Can a use for the prognostic implications of a baseline blood SIRI level in PDAC be envisaged? When considering palliative chemotherapy in the treatment of PDAC there is a difficult balance to achieve between the modest survival benefits and treatment associated deleterious effects on quality of life. Identifying a group of patients with a very poor outcome despite chemotherapy, SIRI > 2.35 less than 20% 1-year survival, may influence patient decisions regarding initiation and duration of treatment. Although cytotoxic chemotherapy is standard first-line treatment in PDAC there remains an urgent need to develop better systemic therapies. However, trials in patients after failure of first-line chemotherapy remain difficult, often as a result of decreased patient fitness, rapid disease progression, and residual toxicity. Patients in whom FOLFIRINOX chemotherapy is unlikely to bring significant benefit may have little to lose by inclusion in first-line clinical studies of novel therapies, potentially as window-of-opportunity trials. In addition, it is recognized that the balance of inflammatory and immune mediators in pancreatic cancer and its associated stroma is associated with prognosis [Citation24]. It is likely, although as yet unproven, that this influences blood inflammatory markers, such as the cells determining SIRI. With the development of therapeutic agents targeting the tumor stroma or associated inflammatory/immune mediators [Citation25] SIRI may allow identification of patients who may benefit more from such novel interventions and their enrichment or stratification within trials.
In conclusion, baseline SIRI before the initiation of FOLFIRINOX for advanced pancreatic cancer can be used as a prognostic marker to identify groups of patients that will differ in their response to chemotherapy with better or worse OS. This marker should be included in future prospective studies as a potential stratification factor.
Supplemental Material
Download MS Word (240.7 KB)Disclosure statement
No potential conflicts of interest were reported by the authors.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA. CA Cancer J Clin. 2020;70(1):7–30.
- Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–2921.
- Sohal DPS, Mangu PB, Laheru D. Metastatic pancreatic cancer: American society of clinical oncology clinical practice guideline summary. J Oncol Pract. 2017;13(4):261–264.
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825.
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-Paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–1703.
- Birnbaum DJ, Bertucci F, Finetti P, et al. Molecular classification as prognostic factor and guide for treatment decision of pancreatic cancer. Biochim Biophys Acta Rev Cancer. 2018;1869(2):248–255.
- McNamara MG, Templeton AJ, Maganti M, et al. Neutrophil/lymphocyte ratio as a prognostic factor in biliary tract cancer. Eur J Cancer. 2014;50(9):1581–1589.
- Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-Lymphocyte ratio in solid tumors: a systematic review and Meta-Analysis. JNCI J Natl Cancer Inst. 2014;106(6):dju124.
- Wang D, Luo H, Qiu M, et al. Comparison of the prognostic values of various inflammation based factors in patients with pancreatic cancer. Med Oncol. 2012;29(5):3092–3100.
- An X, Ding PR, Li YH, et al. Elevated neutrophil to lymphocyte ratio predicts survival in advanced pancreatic cancer. Biomarkers. 2010;15(6):516–522.
- Smith RA, Bosonnet L, Raraty M, et al. Preoperative platelet-lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. Am J Surg. 2009;197(4):466–472.
- Vivaldi C, Caparello C, Musettini G, et al. First-line treatment with FOLFOXIRI for advanced pancreatic cancer in clinical practice: patients’ outcome and analysis of prognostic factors. Int J Cancer. 2016;139(4):938–945.
- Franco F, Camara JC, Martín-Valadés JI, et al. Clinical outcomes of FOLFIRINOX and gemcitabine–nab paclitaxel for metastatic pancreatic cancer in the real world setting. Clin Transl Oncol. 2020;23:812–819.
- Pacheco-Barcia V, Mondéjar Solís R, France T, et al. A systemic inflammation response index (SIRI) correlates with survival and predicts oncological outcome for mFOLFIRINOX therapy in metastatic pancreatic cancer. Pancreatology. 2020;20(2):254–264.
- Qi Q, Zhuang L, Shen Y, et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer. 2016;122(14):2158–2167.
- Kanda M, Fujii T, Kodera Y, et al. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg. 2010;98(2):268–274.
- Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–35.
- Khomiak A, Brunner M, Kordes M, et al. Recent discoveries of diagnostic, prognostic and predictive biomarkers for pancreatic cancer. Cancers (Basel). 2020;12(11):3234.
- Wei L, Xie H, Yan P. Prognostic value of the systemic inflammation response index in human malignancy. Medicine (Baltimore). 2020;99(50):e23486.
- Li S, Xu H, Wang W, et al. The systemic inflammation response index predicts survival and recurrence in patients with resectable pancreatic ductal adenocarcinoma. Cancer Manag Res. 2019;11:3327–3337.
- Chan KKW, Guo H, Cheng S, et al. Real-world outcomes of FOLFIRINOX vs gemcitabine and nab-paclitaxel in advanced pancreatic cancer: a population-based propensity score-weighted analysis. Cancer Med. 2020;9(1):160–169.
- Chllamma MK, Cook N, Dhani NC, et al. FOLFIRINOX for advanced pancreatic cancer: the princess margaret cancer Centre experience. Br J Cancer. 2016;115(6):649–654.
- Topkan E, Mertsoylu H, Kucuk A, et al. Low systemic inflammation response index predicts good prognosis in locally advanced pancreatic carcinoma patients treated with concurrent chemoradiotherapy. Gastroenterol Res Pract. 2020;2020:5701949.
- Lafaro KJ, Melstrom LG. The paradoxical web of pancreatic cancer tumor microenvironment. Am J Pathol. 2019;189(1):44–57.
- Steele CW, Gill NAK, Jamieson NB, et al. Targeting inflammation in pancreatic cancer: clinical translation. World J Gastrointest Oncol. 2016;8(4):380–388.