Abstract
Background
The optimal treatment approach for T4 esophageal cancer is not well established. We aimed to perform a systematic review and meta-analysis to determine the survival rates and safety of chemoradiotherapy followed by surgery (CRT-S) and chemoradiotherapy alone (CRT) in patients with T4 Nany M0 esophageal cancer.
Materials and Methods
We searched databases for eligible prospective or retrospective studies. The outcomes of interest were overall survival (OS) at 1, 3 and 5 years, treatment-related fistula formation and mortality rates. Meta-analyses were performed using the random effects models separately for studies evaluating CRT-S and CRT. Subgroup analyses were performed based on histology, radiation dose, chemotherapy regimen and duration of the interval between CRT and surgery.
Results
We identified 23 studies including 1,119 patients with predominantly squamous cell carcinoma (93%) and adenocarcinoma (3%) histology. The OS rates of patients receiving CRT-S were 65%, 36% and 20% at 1, 3 and 5 years, respectively. The OS rates of patients receiving CRT were 30%, 11% and 10% at 1, 3 and 5 years, respectively. Treatment-related fistula formation rates were 4% for CRT-S and 9% for CRT. Treatment-related mortality rates were 3% for both groups. Subgroup analyses showed that the interval of >2 months between CRT and surgery was associated with significantly improved OS rates at 1, 3 and 5 years.
Conclusion
Chemoradiotherapy is an efficacious treatment approach for T4 esophageal cancer, with clinically acceptable rates of treatment-related fistula formation and mortality. Tri-modality approach with surgery can be considered in carefully selected patients. Our study findings should be interpreted with caution due to the lack of high-quality evidence. Randomized controlled trials are warranted to confirm these findings.
Introduction
The incidence of esophageal cancer is 3.1% among all new cancer cases with five-year survival of up to 30% (AJCC) [Citation1,Citation2]. The incidence of T4 tumors is reported to be 16 to 34% among patients with esophageal cancer [Citation3,Citation4]. T4 esophageal tumors denotes adjacent organ invasion, such as airway (50 to 60%), great vessels (18 to 20%), heart and vertebral body [Citation5,Citation6].
T4 esophageal cancer presents a challenging therapeutic dilemma with no established standard therapy. Multimodality treatment strategy has generally been employed. However, the prognosis is still guarded, because these tumors are technically challenging to resect with clear margins [Citation7,Citation8]. The two widely practiced treatment approaches are chemoradiotherapy followed by surgery (CRT-S) and chemoradiotherapy alone (CRT). Previous prospective studies reported the efficacy of these approaches for T4 esophageal cancer [Citation9–11]. For instance, Fujita et al. reported 5-year overall survival (OS) of 17% with CRT-S and 13% with CRT [Citation9]. These studies are limited by their non-randomized study design and small sample sizes, and the efficacy of these treatment strategies was largely extrapolated from the findings of the landmark trials on locally invasive esophageal cancer. Two randomized controlled trials (RCTs), CROSS trial and RTOG 8501, reported survival benefits with combined modality treatments for locally advanced esophageal cancer [Citation12,Citation13]. However, patients with T4 tumors were excluded from these trials because the eligible patients had to have resectable disease, which is often not the case for most patients with T4 tumors. A Cochrane meta-analysis demonstrated that the addition of surgery to chemoradiotherapy improved local control in patients with T3–T4 and/or node-positive esophageal cancer, but the outcomes for T4 tumors were not reported separately in the included RCTs [Citation14].
Curative treatments of T4 esophageal cancer are associated with high morbidity and mortality. One dreaded treatment complication is fistula formation. Patients with T4-staged tumors are up to 2.6 times more likely to develop an esophageal fistula during or after treatment, compared to early-stage tumors [Citation7,Citation15–17]. Deeper tumor invasion depth, tumor shrinkage during therapy and relatively slower normal tissues repair are the factors that cause the formation of a fistula connecting to the neighboring structures. The overall incidence of fistula formation remains unclear currently. The development of fistula during or after treatment is linked to poor survival outcome [Citation18]. Intercurrent death may occur owing to complications, such as pneumonia due to an esophageal-pulmonary fistula, massive bleeding due to an esophageal-aortic fistula and mediastinitis due to an esophago-mediastinal fistula [Citation7,Citation8].
The previous systematic review was limited by the inclusion of a heterogenous group of patients such as M1 lymph node metastases and the lack of meta-analysis to quantitatively summarize the treatment outcomes [Citation19]. Hence, we aimed to perform a systematic review and meta-analysis to evaluate the survival rates and safety of CRT-S and CRT in T4 esophageal cancer.
Material and methods
Evidence acquisition
The Population, Intervention, Control, Outcomes and Study Design method was used to define literature inclusion criteria (Supplementary Data 1) [Citation20–22]. The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) 2020 and the Meta-analysis of Observational in Epidemiology (MOOSE) reporting guidelines were used [Citation23,Citation24]. A comprehensive search was performed in PUBMED/MEDLINE, Embase and Cochrane Databases from their date of inception to 31 January 2021. The search strategy applied was ‘esophagus’, ‘cancer’, ‘chemoradiotherapy’, ‘surgery’ and ‘T4’ with their synonyms and MeSH terms (Supplementary Data 2). We also performed hand search for additional articles through the reference lists of obtained articles. We used an online software, COVIDENCE for study screening and selection.
Inclusion criteria included prospective or retrospective studies with (1) patients with histologically confirmed clinical T4 Nany M0 esophageal cancer, (2) multi-arm or single-arm study design, (3) all patients in a treatment arm underwent CRT-S or CRT, and (4) at least the primary outcome measure (5-year overall survival) or at least 1 of the secondary outcome measures (incidence of treatment-related fistula formation or treatment-related mortality) was reported. Salvage surgery was allowed and included in the CRT-S treatment group. Exclusion criteria included (1) systematic reviews or case reports, (2) studies that included patients with M1 lymph node metastases, and (3) studies not published in English.
Center for Evidence-Based Medicine levels of evidence were assigned next to each of the included studies [Citation25]. depicts study and patient characteristics; depicts treatment characteristics.
Table 1. Study and patient characteristics.
Table 2. Treatment characteristics.
Outcome measures and data extraction
The primary outcome measures were overall survival (OS) rates at 1, 3 and 5 years. The secondary outcome measures were the rate of treatment-related fistula formation and the rate of treatment-related mortality.
OS was defined from the date of treatment initiation to the date of all-cause death. Studies that defined OS differently or did not provide clear definition of OS were excluded from the meta-analysis. When outcome measure rates were not reported in the article text, Kaplan–Meier curves were digitized using Plot Digitizer (Digitzelt Version 2.5) to extract the pertinent values at 1, 3 and 5 years. This process was performed by two reviewers (CCL and YYS) and discussed with a third reviewer (JT). Treatment-related fistula was defined as the presence of any esophageal-pulmonary fistula, esophageal-aortic fistula and/or esophago-mediastinal fistula that developed during or after any treatment modality including surgery, chemotherapy and radiotherapy. Treatment-related mortality was defined as death directly caused by any treatment modality including surgery, chemotherapy and radiotherapy.
Data extraction was conducted and reviewed by three reviewers independently (CCL, YYS, JT), with consensus attained among the reviewers. Data regarding study, patient, tumor and treatment characteristics were recorded as reported in and Citation2.
Individual study effect sizes were modeled as proportions. The denominator was determined by the total number of patients included for the analysis of the particular outcome measures of interest. The numerator was calculated by multiplying the denominator by the percentage of patients experiencing the respective outcome measure of interest at a prespecified time. For example, if 100 patients were enrolled in a study and the one-year OS rate was 80%, then the numerator would be 100 multiplied by 80%. For each forest plot, the numerator was rounded to the nearest whole number. Each proportion was then expressed as a percentage by dividing the denominator into numerator.
Assessment of methodological quality of included studies
We adopted the Newcastle–Ottawa Scale (NOS) tool to assess the methodologic quality of the eligible studies as we included non-comparative studies in this review [Citation26]. This assessment tool contains three domains: (1) selection, (2) comparability, and (3) outcome. Selection domain assessed the representativeness of the exposed cohort, selection of the non-exposed cohort, ascertainment of exposure and demonstration that outcome of interest was not present at start of study. Comparability domain described if the study controlled for the important confounders, for example in our study, age, pathological complete response, extend of surgical resection and lymph node involvement. Outcome domain comprised of the assessment of outcome, adequacy of follow-up duration for outcomes to occur and adequacy of follow-up of cohorts.
All the included studies were judged by two reviewers (CCL and YYS) using the Newcastle–Ottawa checklist to be low-to-moderate quality (for example, the highest point scored was 6 out of the maximum of 9 points) (Supplementary Data 3).
Statistical analysis
Statistical analyses were performed using R Studio, version 1.4.1106 (R Foundation for Statistical Computing) [Citation27]. The meta-analysis for R (metafor) package, version 2.4-0 and General Package for Meta-Analysis (meta), version 4.13-0 were used to perform the random effects meta-analyses and tests for heterogeneity (I2 and τ2) [Citation28,Citation29]. A 0.5 continuity correction was applied for studies with an event probability of 0 [Citation30,Citation31]. Sensitivity analyses were performed using restricted maximum likelihood method and Knapp–Hartung adjustment model [Citation31]. Weighted random-effect models were used to determine an overall summary estimate for each outcome measure and were depicted on a forest plot with its corresponding 95% confidence interval (CI). A random-effects model was used [Citation32,Citation33]. The R code used to generate each of these analyses is provided in the Supplementary Data 5.
Heterogeneity was assessed using the I2 and τ2 statistics [Citation34,Citation35]. Although heterogeneity was considered significant if I2 was more than 50%, there are limitations of the I2 statistic, such as its high sensitivity to individual study sample sizes; hence, we also provided τ2 to quantify study heterogeneity, which has been calculated using an arcsine transformation, with the value ranging from 0 to τ [Citation36–38].
Subgroup analysis
Subgroup analyses were performed to determine if the results were influenced by: histology (studies that included squamous cell carcinoma (SCC) only versus studies that included other non-SCC histologic types such as adenocarcinoma), radiation dose (>50 Gy vs ≤50 Gy for CRT-S; ≥60 Gy vs < 60 Gy for CRT), chemotherapy regimen (cisplatin-5-fluorouracil (CF) vs non-CF regimen) and interval between chemoradiotherapy and surgery (≤2 months vs >2 months, for CRT-S group). Interaction tests were used to compare differences between estimates from different subgroups.
Results
Study characteristics
The flow diagram of study selection is illustrated in . We excluded eight studies that involved patients with M1 lymph node metastases [Citation8,Citation10,Citation39–44] and three studies that evaluated the outcomes of patients who received other treatment approaches other than CRT-S and CRT [Citation45,Citation46,Citation47].
Twenty-three eligible non-comparative studies comprising 1119 patients were identified (). These included four prospective (two phase II [Citation10,Citation48], one phase I [Citation11] and one prospective cohort studies [Citation9]) and 19 retrospective cohort studies [Citation6,Citation49–66]. The studies were published between 1997 and 2020 as reported in . The countries included Japan [Citation9–11,Citation48–50,Citation52–56,Citation58,Citation59,Citation61,Citation62,Citation65], United States [Citation57], United Kingdom [Citation63], the Netherlands [Citation51], Italy [Citation6], Spain [Citation64], Belgium [Citation60] and China [Citation66]. The median sample size was 43 (range, 18 to 91). Seventeen studies including 564 patients reported on CRT-S [Citation6,Citation9,Citation48–62], whereas 15 studies including 555 patients on CRT [Citation9,Citation10,Citation11,Citation50,Citation51,Citation54,Citation55,Citation58,Citation59,Citation61,Citation62,Citation63,Citation64,Citation65,Citation66]. The study population was comprised of predominantly SCC (92.5%; 1035/1119), followed by adenocarcinoma (3.4%%; 38/1119) and other histologic types (0.3%; 3/1119). The histology of the remaining 43 patients was not reported (3.8%). The tumors were most often located at the middle third of esophagus (20.2%; 266/1119), followed by cervical or upper third (10.6%; 199/1119) and lower third (6.3%; 70/1119). The data of tumor location was unavailable for the remaining 584 patients. The prescribed radiation doses ranged from 30 to 71.4 Gy for CRT-S group and from 50 to 70 Gy for CRT group. Most studies used CF doublet chemotherapeutic regimen [Citation9,Citation11,Citation48–50,Citation52–54,Citation56,Citation58,Citation61–63]. One study administered induction chemotherapy using docetaxel-cisplatin-5-fluorouracil (DCF) followed by concurrent chemoradiotherapy with CF [Citation10].
Among patients who underwent surgical resection after neoadjuvant chemoradiotherapy, the median rates of complete resection (R0) and pathological complete response were 78% (range, 34% to 100%) and 21% (range, 3% to 46%) ().
Overall survival
Thirteen studies defined OS as the duration from the date of treatment initiation to the date of all-cause death [Citation6,Citation9,Citation11,Citation49,Citation51–54,Citation58,Citation62,Citation64–66]. We included these studies into meta-analyses.
Chemoradiotherapy followed by surgery
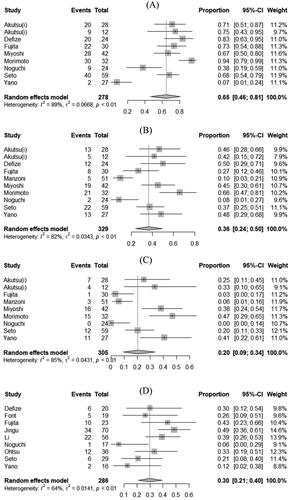
The pooled estimates of 1-, 3- and 5-year OS were 65% (95% CI, 46% to 81%), 36% (95% CI, 24% to 50%) and 20% (95% CI, 9% to 34%) for CRT-S group (). There was significant heterogeneity among the studies for 1-year OS (I2= 80%; τ2= 0.07; p < 0.01), 3-year OS (I2 = 89%; τ2 = 0.03; p < 0.01) and 5-year OS (I2 = 85%; τ2 = 0.04; p < 0.01) outcomes.
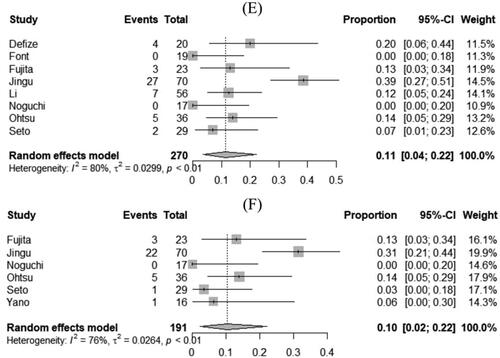
Figure 2. Pooled estimates of overall survival at 1, 3 and 5 years in patients with T4 esophageal cancer treated with chemoradiotherapy followed by surgery (A, B and C, respectively) and chemoradiotherapy alone (D, E and F, respectively). CI: confidence interval.
Chemoradiotherapy alone
The pooled estimates of 1-, 3- and 5-year OS were 30% (95% CI, 21% to 40%), 11% (95% CI, 4% to 22%) and 10% (95% CI, 2% to 22%) for CRT group (). There was significant heterogeneity among the studies for 1-year OS (I2= 64%; τ2 = 0.01; p < 0.01), 3-year OS (I2 = 80%; τ2 = 0.03; p < 0.01) and 5-year OS (I2 = 76%; τ2 = 0.03; p < 0.01) outcomes ().
Rates of treatment-related fistula formation
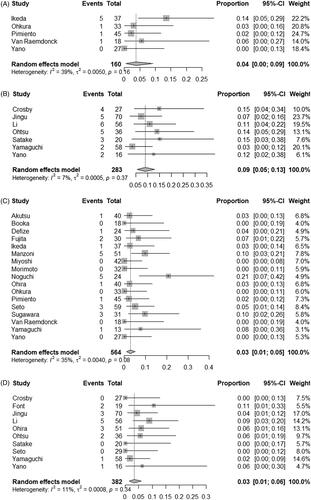
Five studies provided rates of fistula formation related to CRT-S [Citation48,Citation56,Citation57,Citation60,Citation62], whereas seven studies provided rates of fistula formation related to CRT [Citation10,Citation11,Citation61,Citation62,Citation63,Citation65,Citation66]. The pooled estimates of the rates of treatment-related fistula formation were 4% (95% CI, 0% to 9%) in CRT-S group [Citation48,Citation56,Citation57,Citation60,Citation62] and 9% (95% CI, 5% to 13%) in CRT group [Citation10,Citation11,Citation61–63,Citation65,Citation66], respectively (). There was no significant heterogeneity among the studies for treatment-related fistula formation rate outcome in CRT-S (I2 = 39%; τ2 < 0.01; p = 0.16) and CRT (I2= 7%; τ2 < 0.01; p = 0.37) groups.
Rates of treatment-related mortality
Seventeen studies provided rates of treatment-related mortality after CRT-S [Citation6,Citation9,Citation48–62], whereas 10 studies provided rates of fistula formation after CRT [Citation10,Citation11,Citation55,Citation58,Citation61–66]. The pooled estimates of the rates of treatment-related mortality were 3% (95% CI, 1% to 5%) in CRT-S group [Citation6,Citation9,Citation48–62] and 3% (95% CI, 1% to 6%) in CRT group [Citation10,Citation11,Citation55,Citation58,Citation61–66], respectively (). There was no significant heterogeneity among the studies for treatment-related mortality rate outcome in CRT-S (I2= 35%; τ2 < 0.01; p = 0.08) and CRT (I2= 11%; τ2 < 0.01; p = 0.34) groups.
Sensitivity analysis
Sensitivity analyses performed using restricted maximum likelihood method and Knapp-Hartung adjustment model demonstrated similar findings for all the outcomes including 1-, 3- and 5-year OS as well as treatment-related fistula formation and mortality rates (Supplementary Data 4).
For example, using restricted maximum likelihood method, the pooled estimates of 1-year OS were 65% (95% CI, 45% to 82%) for CRT-S group and 30% (95% CI, 21% to 40%) for CRT group. Using Knapp–Hartung adjustment model, the pooled estimates of 1-year OS were 65% (95% CI, 42% to 85%) for CRT-S group and 30% (95% CI, 19% to 42%) for CRT group (Supplementary Data 4).
Subgroup analysis
Subgroup analyses showed that there were no significant effect modifications by histology, radiation dose and chemotherapy regimen. In CRT-S group, patients with the interval of >2 months between CRT and surgery had significantly improved OS rates at 1, 3 and 5 years, compared to those with the interval of ≤2 months (1-year, 90% vs 73%; 3-year, 59% vs 37%; 5-year, 47% vs 17%). There was also significant improvement in the 5-year OS rates with the use of radiation dose of ≤50 Gy (26% vs 6%) and CF regimen (23% vs 6%) in the CRT-S group (Supplementary Data 5). Compared to the studies published before 2010, those published later had significantly improved OS rates at 1, 3 and 5 years for CRT-S group (1-year, 83% vs 50%; 3-year, 53% vs 28%; 5-year, 35% vs 15%), and at 1 and 5 years for CRT group (1-year, 42% vs 24%; 5-year, 31% vs 7%).
Discussion
This systematic review and meta-analysis using the best available evidence summarized the outcomes of patients who received CRT-S and CRT for non-metastatic T4 esophageal cancer. The pooled estimates of 1-, 3- and 5-year OS were 65%, 36% and 20% for CRT-S group, and 30%, 11% and 10% for CRT group. The rates of treatment-related fistula formation were 4% in CRT-S and 9% in CRT. The rates of treatment-related deaths were similarly low at 3% between two treatment groups.
The previous reviews suggested that CRT-S was superior to CRT with respect to local disease control and short-term survival but was associated with relatively increased perioperative mortality and morbidity [Citation19,Citation67]. However, these reviews were limited by the absence of comprehensive search strategy, methodologic quality assessment of the included studies and meta-analysis. In addition, the overall patient population in the review was heterogeneous and included studies with patients with M1 lymph node metastases such as cervical lymph node. In our review, we included only studies with non-metastatic T4 disease and four new studies through the updated search [Citation46,Citation50,Citation56,Citation61] and performed meta-analysis to estimate the pooled outcomes.
Based on our subgroup analysis, we observed that patients with longer interval between CRT and surgery of more than 2 months had significantly improved OS rates at 1, 3 and 5 years, compared to those with shorter interval. Data on esophageal and gastroesophageal cancers in the CROSS study showed 23.6% complete histological response in patients operated <8 weeks after CRT and 43.1% in patients operated >8 weeks after CRT [Citation12]. Complete pathologic response rate has been shown to predict superior OS [Citation68]. There is an increasing body of data from the treatment of other primary malignancies such as rectal cancer indicating that the timing of surgery after CRT may have a strong beneficial impact on the tumor response after CRT [Citation69,Citation70]. However, a meta-analysis including 12621 esophageal cancer patients from 16 cohort studies demonstrated that patients with a longer time interval between neoadjuvant CRT and esophagectomy had significantly worse OS [Citation71]. Thus, the prognostic role of the time interval between CRT and surgery in esophageal cancer is still controversial.
Our study demonstrated that patients treated with CRT-S had lower rate of fistula formation, compared to those treated with CRT (4% vs 9%). There are several possible explanations. This might be due to patient selection, where those patients with fistula formation before or during chemoradiotherapy may not be suitable for surgery. Moreover, majority of the studies are retrospective in nature and the rate of fistula complication may be underreported. Our study also demonstrated that studies that the use radiation dose of 50 Gy and less, CF chemotherapy regimen and interval duration of more than 2 months between CRT and surgery were associated with improved OS rates. These findings are hypothesis generating. One possible explanation is that the tumor burden in these studies may be lower which may influence the investigators to use lower radiation dose as well as older chemotherapy regimens and prolong the duration between CRT and surgery.
This study has several strengths. Firstly, we focused exclusively on patients with T4 esophageal cancer without distant metastases. Secondly, we used robust meta-analysis techniques and sensitivity analytic models to calculate the pooled estimates of outcome data. Thirdly, we performed subgroup analyses to determine whether the outcomes were influenced by histology, radiation dose, chemotherapy regimen and interval to surgery. Fourthly, we employed a validated quality assessment tool, namely Newcastle–Ottawa scale, to assess the methodologic quality of the eligible studies.
The limitations of our study include, firstly, statistical comparison was not possible between both treatment groups given the potential patient selection bias where patients with longer life expectancy and limited medical comorbidities were more likely to have undergone surgery. Secondly, there was significant heterogeneity across studies and patient population. The majority of the publications were small and retrospective. We are not able to perform more granular analyses because of the lack of individual patient data. Thirdly, the included studies in our review were largely performed in Asian countries with predominantly SCC histologic subtype; hence, our study findings may not be generalized to the non-Asian population with typically adenocarcinoma since this epidemiologic disparity has been reported in the literature [Citation72]. Fifthly, the lack of information on follow-up time and non-systematic follow-up increases the risk of data underreporting and is crucial for observations of our key endpoints such as OS and fistula. Other late effects such as esophageal stricture, the need for permanent feeding tube, subsequent heart and lung toxicities are relevant for QOL but not adequately reported.
This study has important implications on this research area where the knowledge on the treatment outcomes is still limited. Our study provides useful information including estimated survival benefit and risk quotation which helps clinicians in making treatment recommendations during consultation so that patients can make informed choices on therapy options. Both treatment approaches are safe and efficacious. Careful patient selection for surgery is an acceptable option. While the outcomes of T4 esophageal cancer treated with conventional approaches have been unchanged over past decades and a recent phase III RCT (ARTDECO) showed that definitive CRT with dose escalation up to 61.6 Gy did not result in a significant increase in local control over 50.4 Gy [Citation73], future trials should consider incorporating novel cancer therapies, such as immune checkpoint inhibitors and modern radiotherapy techniques as part of the multimodality treatment strategies. A phase-III placebo-controlled trial has demonstrated that adjuvant nivolumab significantly improved disease-free survival in patients with resected locally advanced esophageal cancer [Citation74]. Proton beam therapy has been shown to confer dosimetric superiority which may translate into reduction of clinically significant toxicities in the treatment of locally advanced esophageal cancer [Citation75]. We also suggest the incorporation of biomarkers into prognostication model in future clinical trials to allow better patient selection for radical treatment. For example, Glasgow Prognostic Score (GPS) based on C-reactive protein and serum albumin has been proposed to be a useful prognostication tool in patients with T4 esophageal cancer [Citation55].
In conclusion, chemoradiotherapy alone is an efficacious treatment approach for T4 esophageal cancer, with clinically acceptable rates of treatment-related fistula formation and mortality. Tri-modality approach combining chemoradiotherapy with surgery is a clinically beneficial option in carefully selected patients. However, our study findings should be interpreted with caution due to the lack of high-quality evidence. Randomized controlled trials are warranted to confirm these findings.
Supplemental Material
Download MS Word (56.5 KB)Acknowledgement
The authors thank our department for their support and assistance throughout all aspects of our study.
Disclosure statement
All authors did not have conflict of interest to disclose.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249.
- Rice TW, Patil DT, Blackstone EH. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann Cardiothorac Surg. 2017;6(2):119–130.
- Holscher AH, Bollschweiler E, Bumm R, et al. Prognostic factors of resected adenocarcinoma of the esophagus. Surgery. 1995;118(5):845–855.
- Tabira Y, Yasunaga M, Nakano KY, et al. All pathological T4 esophageal carcinomas should be categorized as stage IV. Hepatogastroenterology. 2002;49(45):694–698.
- Fockens P, Kisman K, Merkus MP, et al. The prognosis of esophageal carcinoma staged irresectable (T4) by endosonography. J Am Coll Surg. 1998;186(1):17–23.
- de Manzoni G, Pedrazzani C, Pasini F, et al. Chemoradiotherapy followed by surgery for squamous cell carcinoma of the thoracic esophagus with clinical evidence of adjacent organ invasion. J Surg Oncol. 2007; 95(3):261–266.
- Chen HY, Ma XM, Ye M, et al. Esophageal perforation during or after conformal radiotherapy for esophageal carcinoma. J Radiat Res. 2014;55(5):940–947.
- Shinoda M, Ando N, Kato K, Japan Clinical Oncology Group, et al. Randomized study of low-dose versus standard-dose chemoradiotherapy for unresectable esophageal squamous cell carcinoma (JCOG0303). Cancer Sci. 2015;106(4):407–412.
- Fujita H, Sueyoshi S, Tanaka T, et al. Esophagectomy: is it necessary after chemoradiotherapy for a locally advanced T4 esophageal cancer? Prospective nonrandomized trial comparing chemoradiotherapy with surgery versus without surgery. World J Surg. 2005;29(1):25–30. discussion 30–1.
- Satake H, Tahara M, Mochizuki S, et al. A prospective, multicenter phase I/II study of induction chemotherapy with docetaxel, cisplatin and fluorouracil (DCF) followed by chemoradiotherapy in patients with unresectable locally advanced esophageal carcinoma. Cancer Chemother Pharmacol. 2016;78(1):91–99.
- Ohtsu A, Boku N, Muro K, et al. Definitive chemoradiotherapy for T4 and/or M1 lymph node squamous cell carcinoma of the esophagus. J Clin Oncol. 1999;17(9):2915–2921.
- Shapiro J, van Lanschot JJB, Hulshof M, CROSS study group, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16(9):1090–1098.
- Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). radiation therapy oncology group. JAMA. 1999; May 5281(17):1623–1627.
- Vellayappan BA, Soon YY, Ku GY, et al. Chemoradiotherapy versus chemoradiotherapy plus surgery for esophageal cancer. Cochrane Database Syst Rev. 2017;8:CD010511.
- Xu Y, Wang L, He B, et al. Development and validation of a risk prediction model for radiotherapy-related esophageal fistula in esophageal cancer. Radiat Oncol. 2019;14(1):181.
- Zhu C, Wang S, You Y, et al. Risk factors for esophageal fistula in esophageal cancer patients treated with radiotherapy: a systematic review and Meta-Analysis. Oncol Res Treat. 2020;43(1-2):34–41.
- Zhang Y, Li Z, Zhang W, et al. Risk factors for esophageal fistula in patients with locally advanced esophageal carcinoma receiving chemoradiotherapy. Onco Targets Ther. 2018;11:2311–2317.
- Kim H, Oh D, Ahn YC, et al. Clinical outcomes of radiation therapy for clinical T4b oesophageal cancer with airway invasion. Radiat Oncol. 2018;13(1):245.
- Makino T, Yamasaki M, Tanaka K, et al. Treatment and clinical outcome of clinical T4 esophageal cancer: a systematic review. Ann Gastroenterol Surg. 2019;3(2):169–180.
- Richardson WS, Wilson MC, Nishikawa J, et al. The well-built clinical question: a key to evidence-based decisions. ACP J Club. 1995; 123(3):A12–3.
- Ebell M. Information at the point of care: answering clinical questions. J Am Board Fam Pract. 1999; 12(3):225–235.
- Huang X, Lin J, Demner-Fushman D. Evaluation of PICO as a knowledge representation for clinical questions. AMIA Annu Symp Proc. 2006;2006:359–363.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
- Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012.
- J H. Levels of evidence. Oxford Centre for Evidence-Based Medicine. March 2009.
- Wells G, Shea B. D OC. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses 2013. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- Studio R. Integrated development environment for R. 2015. Accessed 25 February, 2020. https://rstudio.com/products/rstudio/.
- Viechtbauer W. Conducting Meta-analyses in R with the metafor package. J Statistical Software. 2010;36(3):1–48.
- Balduzzi S, Rucker G, Schwarzer G. How to perform a Meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153–160.
- Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Statist. 1950;21(4):607–611.
- Langan D, Higgins JPT, Jackson D, et al. A comparison of heterogeneity variance estimators in simulated random-effects meta-analyses. Res Synth Methods. 2019;10(1):83–98.
- Ades AE, Lu G, Higgins JP. The interpretation of random-effects Meta-analysis in decision models. Med Decis Making. 2005; 25(6):646–654.
- Fleiss JL, Gross AJ. Meta-analysis in epidemiology, with special reference to studies of the association between exposure to environmental tobacco smoke and lung cancer: a critique. J Clin Epidemiol. 1991;44(2):127–139.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558.
- Wg C. The combination of estimates from different experiments. Biometrics. 1954;10(1):101–129.
- Rucker G, Schwarzer G, Carpenter JR, et al. Undue reliance on I(2) in assessing heterogeneity may mislead. BMC Med Res Methodol. 2008;8:79.
- Serghiou S, Goodman SN. Random-effects meta-analysis: summarizing evidence with caveats. JAMA. 2019;321(3):301–302.
- IntHout J, Ioannidis JP, Rovers MM, et al. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. 2016;6(7):e010247.
- Ishida K, Ando N, Yamamoto S, et al. Phase II study of cisplatin and 5-fluorouracil with concurrent radiotherapy in advanced squamous cell carcinoma of the esophagus: a Japan esophageal oncology group (JEOG)/Japan clinical oncology group trial (JCOG9516). Jpn J Clin Oncol. 2004;34(10):615–619.
- Kaneko K, Ito H, Konishi K, et al. Definitive chemoradiotherapy for patients with malignant stricture due to T3 or T4 squamous cell carcinoma of the oesophagus. Br J Cancer. 2003;88(1):18–24.
- Miyazaki T, Sohda M, Tanaka N, et al. Phase I/II study of docetaxel, cisplatin, and 5-fluorouracil combination chemoradiotherapy in patients with advanced esophageal cancer. Cancer Chemother Pharmacol. 2015;75(3):449–455.
- Nishimura Y, Suzuki M, Nakamatsu K, et al. Prospective trial of concurrent chemoradiotherapy with protracted infusion of 5-fluorouracil and cisplatin for T4 esophageal cancer with or without fistula. Int J Radiat Oncol Biol Phys. 2002;53(1):134–139.
- Ohtsu A, Yoshida S, Boku N, et al. Concurrent chemotherapy and radiation therapy for locally advanced carcinoma of the esophagus. Jpn J Clin Oncol. 1995;25(6):261–266.
- Yokota T, Kato K, Hamamoto Y, et al. Phase II study of chemoselection with docetaxel plus cisplatin and 5-fluorouracil induction chemotherapy and subsequent conversion surgery for locally advanced unresectable oesophageal cancer. Br J Cancer. 2016;115(11):1328–1334.
- Shimoji H, Karimata H, Nagahama M, et al. Induction chemotherapy or chemoradiotherapy followed by radical esophagectomy for T4 esophageal cancer: results of a prospective cohort study. World J Surg. 2013;37(9):2180–2188.
- Okamura A, Hayami M, Kozuki R, et al. Salvage esophagectomy for initially unresectable locally advanced T4 esophageal squamous cell carcinoma. Esophagus. 2020;17(1):59–66.
- Miyata H, Yamasaki M, Kurokawa Y, et al. Clinical relevance of induction triplet chemotherapy for esophageal cancer invading adjacent organs. J Surg Oncol. 2012;106(4):441–447.
- Ikeda K, Ishida K, Sato N, et al. Chemoradiotherapy followed by surgery for thoracic esophageal cancer potentially or actually involving adjacent organs. Dis Esophagus. 2001;14(3-4):197–201.
- Akutsu Y, Matsubara H. Chemoradiotherapy and surgery for T4 esophageal cancer in Japan. Surg Today. 2015;45(11):1360–1365.
- Booka E, Haneda R, Ishii K, et al. Appropriate candidates for salvage esophagectomy of initially unresectable locally advanced T4 esophageal squamous cell carcinoma. Ann Surg Oncol. 2020;27(9):3163–3170.
- Defize IL, van der Horst S, Bulbul M, et al. Salvage Robot-Assisted minimally invasive esophagectomy (RAMIE) for T4b esophageal cancer after definitive chemoradiotherapy. Ann Surg Oncol. 2021;28(5):2730–2738.
- Miyoshi N, Yano M, Takachi K, et al. Myelotoxicity of preoperative chemoradiotherapy is a significant determinant of poor prognosis in patients with T4 esophageal cancer. J Surg Oncol. 2009;99(5):302–306.
- Morimoto H, Fujiwara Y, Lee S, et al. Treatment results of neoadjuvant chemoradiotherapy followed by radical esophagectomy in patients with initially inoperable thoracic esophageal cancer. Jpn J Radiol. 2018;36(1):23–29.
- Noguchi T, Moriyama H, Wada S, et al. Resection surgery with neoadjuvant chemoradiotherapy improves outcomes of patients with T4 esophageal carcinoma. Dis Esophagus. 2003;16(2):94–98.
- Ohira M, Kubo N, Masuda G, et al. Glasgow prognostic score as a prognostic clinical marker in T4 esophageal squamous cell carcinoma. Anticancer Res. 2015;35(9):4897–4901.
- Ohkura Y, Ueno M, Iizuka T, et al. Prognostic factors and appropriate lymph node dissection in salvage esophagectomy for locally advanced T4 esophageal cancer. Ann Surg Oncol. 2019;26(1):209–216.
- Pimiento JM, Weber J, Hoffe SE, et al. Outcomes associated with surgery for T4 esophageal cancer. Ann Surg Oncol. 2013;20(8):2706–2712.
- Seto Y, Chin K, Gomi K, et al. Treatment of thoracic esophageal carcinoma invading adjacent structures. Cancer Sci. 2007;98(7):937–942.
- Sugawara K, Yagi K, Okumura Y, et al. Long-term outcomes of multimodal therapy combining definitive chemoradiotherapy and salvage surgery for T4 esophageal squamous cell carcinoma. Int J Clin Oncol. 2020;25(4):552–560.
- Van Raemdonck D, Van Cutsem E, Menten J, et al. Induction therapy for clinical T4 oesophageal carcinoma; a plea for continued surgical exploration. Eur J Cardiothorac Surg. 1997; 1(5):828–837.
- Yamaguchi S, Morita M, Yamamoto M, et al. Long-Term outcome of definitive chemoradiotherapy and induction chemoradiotherapy followed by surgery for T4 esophageal cancer with tracheobronchial invasion. Ann Surg Oncol. 2018;25(11):3280–3287.
- Yano M, Tsujinaka T, Shiozaki H, et al. Concurrent chemotherapy (5-fluorouracil and cisplatin) and radiation therapy followed by surgery for T4 squamous cell carcinoma of the esophagus. J Surg Oncol. 1999;70(1):25–32.
- Crosby TD, Brewster AE, Borley A, et al. Definitive chemoradiation in patients with inoperable oesophageal carcinoma. Br J Cancer. 2004;90(1):70–75.
- Font A, Arellano A, Fernandez-Llamazares J, et al. Weekly docetaxel with concomitant radiotherapy in patients with inoperable oesophageal cancer. Clin Transl Oncol. 2007;9(3):177–182.
- Jingu K, Umezawa R, Matsushita H, et al. Chemoradiotherapy for T4 and/or M1 lymph node esophageal cancer: experience since 2000 at a high-volume center in Japan. Int J Clin Oncol. 2016;21(2):276–282.
- Li M, Zhao F, Zhang X, et al. Involved-field irradiation in definitive chemoradiotherapy for T4 squamous cell carcinoma of the esophagus. Curr Oncol. 2016;23(2):e131–e137.
- Makino T, Doki Y. Treatment of T4 esophageal cancer. Definitive chemo-radiotherapy vs chemo-radiotherapy followed by surgery. Ann Thorac Cardiovasc Surg. 2011;17(3):221–228.
- Soror T, Kho G, Zhao KL, et al. Impact of pathological complete response following neoadjuvant chemoradiotherapy in esophageal cancer. J Thorac Dis. 2018;10(7):4069–4076.
- Perez RO, Habr-Gama A, Sao Juliao GP, et al. Optimal timing for assessment of tumor response to neoadjuvant chemoradiation in patients with rectal cancer: do all patients benefit from waiting longer than 6 weeks? Int J Radiat Oncol Biol Phys. 2012;84(5):1159–1165.
- Garcia-Aguilar J, Smith DD, Avila K, Timing of Rectal Cancer Response to Chemoradiation Consortium, et al. Optimal timing of surgery after chemoradiation for advanced rectal cancer: preliminary results of a multicenter, nonrandomized phase II prospective trial. Ann Surg. 2011;254(1):97–102.
- Shang QX, Yang YS, Gu YM, et al. Timing of surgery after neoadjuvant chemoradiotherapy affects oncologic outcomes in patients with esophageal cancer. WJGO. 2020;12(6):687–698.
- Zhang HZ, Jin GF, Shen HB. Epidemiologic differences in esophageal cancer between asian and Western populations. Chin J Cancer. 2012;31(6):281–286.
- Hulshof M, Geijsen ED, Rozema T, et al. Randomized study on dose escalation in definitive chemoradiation for patients with locally advanced esophageal cancer (ARTDECO study). J Clin Oncol. 2021;39(25):2816–2824.
- Kelly RJ, Ajani JA, Kuzdzal J, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med. 2021;384(13):1191–1203.
- Lin SH, Hobbs BP, Verma V, et al. Randomized phase IIB trial of proton beam therapy versus Intensity-Modulated radiation therapy for locally advanced esophageal cancer. J Clin Oncol. 2020; 38(14):1569–1579.