Abstract
Purpose
There is increasing interest in using stereotactic body radiation therapy (SBRT) in areas of oligoprogressive metastatic disease (OPD). Our main objective was to investigate the impact of SBRT on overall survival (OS) and the incidence of systemic therapy treatment switches in this population.
Methods
A retrospective institutional review of patients treated with SBRT for OPD was performed. Patients were included if they received SBRT for 1–3 discrete progressing metastases, using a dose of at least 5 Gy per fraction. The study aimed to calculate progression-free survival (PFS), overall survival (OS), local control (LC), and incidence of treatment switch (TS). PFS and OS were calculated using the Kaplan–Meier methodology, while LC and TS were determined using cumulative incidence.
Results
Eighty-one patients with a total of 118 lesions were treated with SBRT from July 2014 to November 2020. The Median SBRT dose was 40 (18–60) Gy in 5 (2–8) fractions. Patients had primarily kidney, lung, or breast cancer. Most patients were treated with a tyrosine kinase inhibitor (TKI) (30.9%) or chemotherapy (29.6%) before OPD. The median follow-up post-SBRT was 14 months. Median OS and PFS were 25.1 (95% CI 11.2–39.1) months and 7.8 (95% CI 4.6–10.9) months, respectively. The cumulative incidence of local progression of treated lesions was 5% at 1 year and 7.3% at 2 years. Sixty patients progressed after SBRT and 17 underwent additional SBRT. Thirty-eight patients (47%) changed systemic therapy following SBRT; the cumulative incidence of TS was 28.5% at 6 months, 37.4% at 1 year, and 43.9% at 2 years.
Conclusions
SBRT effectively controls locally progressing lesions but distant progression still occurs frequently. A sizeable number of patients can be salvaged by further SBRT or have minimally progressing diseases that may not warrant an immediate initiation/switch in systemic therapy. Further prospective studies are needed to validate this benefit.
Introduction
Oligoprogressive disease (OPD) is a relatively new clinical concept that has been garnering increasing interest in recent years. This term is distinct from that of oligometastatic disease as originally described by Hellman and Weichselbaum [Citation1], which refers to patients who have metastatic disease that is limited in the overall number of sites, typically described as up to five lesions. This leads to oligometastatic disease being defined as an intermediate state between localized and widespread disease [Citation2]. OPD on the other hand is a more specific clinical scenario that occurs when patients are either on systemic therapy or surveillance for more widely spread metastatic disease. After initial stability or response to treatment, disease progression then occurs in only a limited number of sites with a stable response at the other sites [Citation3,Citation4].
The reason for this variable response to systemic therapy is hypothesized to be related to inter and intratumor heterogeneity and the development of drug-resistant subclones [Citation5]. This has been investigated by Gerlinger et al. in patients with NSCLC where biopsies of the primary and metastatic disease sites demonstrated significant genetic heterogeneity [Citation6,Citation7]. The mechanisms behind this variation were discovered to be related to changes in receptor and signaling pathways as investigated by Campo et al. [Citation8]. With the increased usage of oncogene-directed treatment, as well as immunotherapy, the variation between metastatic sites has significant implications for the effectiveness of treatment paradigms [Citation9]. When patients progress on their systemic therapy there are essentially three reasonable treatment options described in the literature—changing the systemic therapy, continuing the same systemic therapy beyond progression, or using local therapy to eradicate the treatment-resistant clones [Citation10,Citation11].
It is through this lens that the role of radiotherapy in the treatment of OPD has arisen. Using stereotactic body radiation therapy (SBRT), the progressing sites can theoretically be targeted and ablated. The number of progressing sites has typically been described as up to three to five lesions [Citation1,Citation3,Citation5] in a paradigm like an oligometastatic disease. The main purpose would be to delay the need for a systemic therapy regimen switch [Citation12]. Targeting sites of OPD with SBRT has already been introduced as part of the management of patients with non-small cell lung cancer (NSCLC) and is included in clinical practice guidelines as a viable treatment strategy [Citation13,Citation14]. However, further work is needed to demonstrate the benefits of this treatment approach. Preliminary studies in NSCLC, prostate and renal cell cancers have found that the ablative treatments for OPD are safe with minimal toxicities [Citation15–17], and may lead to improved disease control.
The purpose of the current study was to perform a single institution review of cancer patients with OPD treated with SBRT. The main objective of this work was to determine if SBRT would provide a tangible benefit in terms of delaying overall progression, controlling resistant metastatic lesions, and increasing the time to therapy switch.
Methodology
Study design
A retrospective chart review of oncology patients was conducted at a large Canadian academic center following institutional ethics approval. Using an institutional database query search all patients who received SBRT at a minimum of 5 Gy per fraction treated from January 2009 to November 2020 were identified. Clinical records were reviewed, and all patients defined to have OPD with extracranial metastasis were identified. The inclusion criteria of the study were as follows: (1) Patient received SBRT for Extracranial OPD. (2) Patients must have 1–5 (no identified patients had >3) oligoprogressive metastasis, defined as progressive disease detected with either CT, MRI, PET, or bone scan (depending on the clinical scenario). (3) Patients must have been treated with SBRT with a dose of at least 5 Gy per fraction. Exclusion criteria were as follows: (1) Intracranial Oligoprogressive Metastasis, (2) Patients who were diagnosed with OPD but did not receive SBRT treatment.
Clinical criteria
All SBRT was given according to institutional standards for each given disease site and dose, technique, and organ-at-risk tolerances based on established institutional protocols [Citation18,Citation19]. Baseline clinical parameters were determined through retrospective collection of electronic records including patient age, sex, primary disease, treatment characteristics, and details of patient follow-up.
Operationally, the outcome of progression-free survival (PFS) was defined as the time between the end of SBRT treatment and the identification of the first local or distant progression, or death. For additional outcomes, overall survival (OS) was measured from the end of SBRT treatment to the last follow-up or death. The period between the end of SBRT treatment and the date of the switch (or start) of the systemic therapy regimen was used for the incidence to switch systemic therapy treatment. Patient and lesion response to SBRT treatment was defined utilizing the RECIST 1.1 criteria [Citation20]. Responses were categorized as Complete Response (CR), Partial Response (PR), Progressive Disease (PD), and Stable Disease (SD) based on routine imaging findings on patient follow-up, as per disease site, and standard clinical care practices.
Toxicity
Adverse SBRT Toxicities were retrospectively collected and scored according to the RTOG and EORTC toxicity criteria for radiation therapy [Citation21]. Acute Toxicity was defined as symptoms presenting within 3 months and late toxicity was defined as symptoms presenting >3 months.
Statistical analysis
The main outcome studied was PFS after SBRT. Important additional outcomes included incidence of systemic therapy treatment switch (TS), OS, local control, and grade of toxicity. Kaplan–Meier analysis was carried out for the overall and progression-free survival outcomes. while local control and treatment switch was determined using cumulative incidence to account for competing risks of death without progression. Additionally, the following statistics were performed: a) a univariate and multivariate cox regression model with PFS as the main outcome of interest and b) a comparison of the cumulative incidence of treatment switch based on predictors identified using the cox regression analysis. All analyses were performed using Microsoft Excel 365, and IBM SPSS v. 20.
Results
Patient information
A full set of patient information can be seen in . Overall, 81 patients were identified with OPD metastatic disease treated from July 2014 to November 2020. The majority of patients were ECOG 0 or 1. Most patients had Renal cell carcinoma (RCC), NSCLC, breast, and colorectal cancer. Patients presented with a wide variation in the initial stage at presentation, and variable time from initial diagnosis of metastatic disease to the development of OPD, with a median time of 24 months (range 0–108 months).
Table 1. Baseline patient characteristics.
Oligoprogressive disease and SBRT characteristics
The oligoprogression and SBRT characteristics can be seen in and . The majority of patients were on either Tyrosine Kinase Inhibitor (TKI) therapy, chemotherapy, or immunotherapy before their OPD. Ninety-four percent of patients received systemic therapy for metastatic disease. Moreover, ten percent of patients had RCC, thyroid cancer, or sarcoma and were on surveillance for indolently progressing metastatic disease. These patients were still considered to have OPD despite having never been previously treated with systemic therapy, as previous data has supported active surveillance in these patient populations [Citation22,Citation23].
Table 2. Systemic therapy and oligoprogressive disease characteristics.
Table 3. Metastasis and SBRT details.
A total of 118 metastases received SBRT. The median SBRT dose was 40 (18–60) Gy in 5 (2–8) fractions. There was a wide variation in the sites of progressing metastasis with over 50% occurring as lung or liver metastases. Of note, however, is the fact that ∼29% of the cohort had SBRT, surgery, or both for initial oligometastatic disease, before oligoprogression.
Clinical outcomes
The median follow-up post-SBRT was noted to be 14 months. and depict PFS and OS outcomes. Median PFS in the entire cohort was 7.8 months (95%CI 4.6–10.9). Median OS was 25.1 months (95% CI 11.2–39.1). In terms of patterns of progression, most patients progressed distantly following SBRT, with a total of 56 patients having progressed distantly, 5 with isolated local progression, and one patient with progression both locally and distantly. The cumulative incidence of local failure can be seen in and was 5% at 1 year and 7.3% at 2 years.
Figure 1. Kaplan–Meier analysis of overall survival in oligoprogressive metastatic disease. Median overall survival was noted to be 25.1 months.
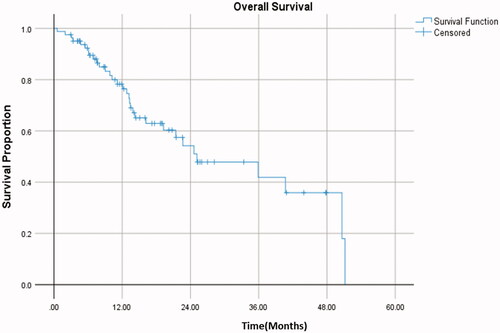
Figure 2. Kaplan–Meier analysis of progression free survival in oligoprogressive metastatic disease. Median progression free survival was 7.8 months.
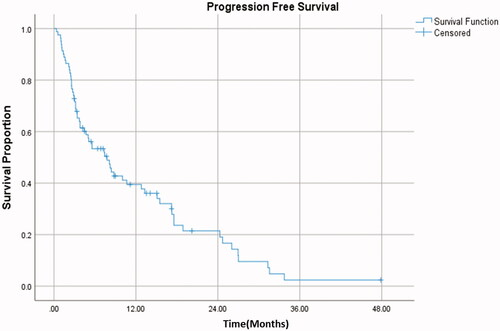
Figure 3. Cumulative incidence demonstrating the proportion of patients with local failure after SBRT over time. Five patients had local progression following SBRT treatment.
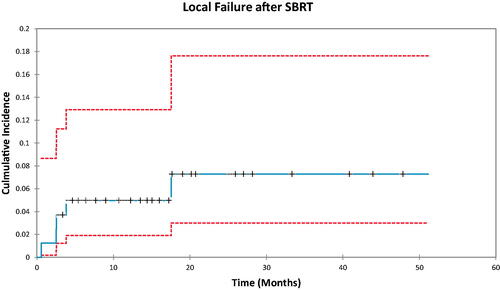
The systemic therapy treatment switch analysis is seen in . The incidence of patients who underwent TS was 28.5% at 6 months (95%CI 20–40.6), 37.4% at 1 year (95%CI 27.8–50.3), and 43.9% at 2 years (95%CI 33.3–58.0). In total, 38 patients (47%) had a switch in systemic treatment following first or subsequent progression. Moreover, 17 patients received additional SBRT for further OPD, with three of them (21%) not requiring a subsequent TS.
Figure 4. Cumulative incidence demonstrating the proportion of patients who had a switch in systemic therapy treatment over time.
Finally, toxicity data can be seen in . No patients experienced grade 4 or 5 acute or late toxicities. Over half of the patients did not experience any acute toxicity and those who did primarily experienced fatigue, pain, or nausea/vomiting, all of which were self-limiting. Only 1 patient experienced late toxicity due to SBRT.
Table 4. Toxicity characteristics.
demonstrates the results of our multivariate cox regression analysis. Of note, a univariate analysis was performed and the only significant factor was the time to oligoprogression after the initial diagnosis of metastatic disease. This was reflected as the main statistically significant result in the Cox multivariate analysis. For the melanoma subgroup although there was noted to be a significant difference there were only six patients in this cohort which could significantly skew the result. Of note, there was also an increasing trend as the number of treated organs increased.
Table 5. Multivariate model for predictors of progression-free survival.
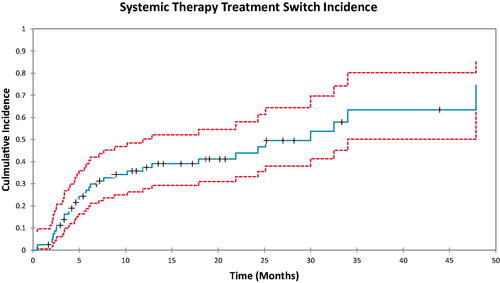
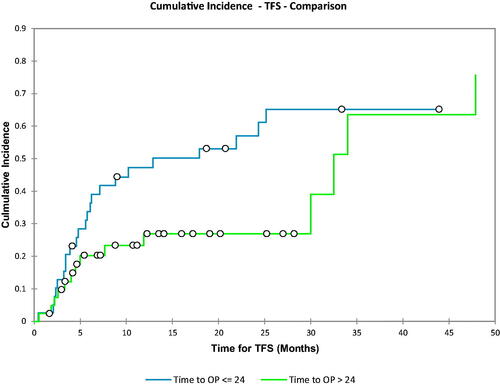
In , a Gray test was performed for the Cumulative incidence utilizing the time to oligoprogression as a dichotomous variable around the median value. The comparison for the test looked at the cumulative incidence of treatment switch for an oligoprogression time less than or equal to the median (24 months) as compared to the time to oligoprogression >24 months. The p-value for this analysis was 0.068. It can also be seen that at 1 year the CI for treatment switch is ∼53% for the group that had a time to oligoprogression <24 months, whereas it is 27% for the group which had a time to oligoprogression >24 months.
Figure 5. Comparison of the cumulative incidence of treatment switch based on the cox regression analysis. Group 1 was the time to oligoprogression less than or equal to the median (24 months). Group 2 was defined as the time to oligoprogression greater than the median (24 months). The p-value in this case was 0.068.
Discussion
This retrospective study investigated eighty-one patients with a total of 118 lesions that were treated with SBRT for OPD. In this work, a variety of disease sites and systemic therapy strategies were included. The goal was to look not only at PFS and OS but also investigate the effect that SBRT can have on the time to switch systemic therapy.
The interpretation of the current studies results requires two key considerations. The first is that a considerable portion of the literature on OPD is directed toward NSCLC. However, in this study, only 17% of the identified patients had NSCLC, with RCC, breast, and colorectal cancers making up over 50% of patients. Secondly, since a variety of disease sites were investigated there was a variation in the systemic therapy that patients were receiving before oligoprogression. This heterogeneity in the disease sites and systemic therapy regimens used does require a broader interpretation of the treatment of OPD in general.
Nonetheless, to appreciate the literature in the field a look at NSCLC is beneficial. In a comparison of the findings from various studies can be seen. In NSCLC a review study found that PFS has varied between 5.5 and 10 months in various studies [Citation11]. Two of the largest studies performed included 206 and 106 patients. Xu et al. [Citation24] found a median PFS of 7.6 months and an OS of 37.4 months. Borghetti et al. [Citation25] found an overall survival of 23 months. A study by Weickhardt et al. [Citation15] investigated intracranial or extracranial oligoprogressive disease and found a progression-free survival after local therapy of 6.2 months. However, the patients with intracranial oligoprogression had a median PFS of 7.1 months whereas those with the extracranial disease had a median PFS of 4.0 months. This suggests there may be additional value in separate investigation and analysis of patients with intracranial oligoprogressive disease. The patients in this current study were only treated for extracranial OPD. Additionally, in the current OPD patient population, there may be greater clinical relevance for TS and prolongation of systemic therapy as endpoints, as opposed to more traditional PFS measures. Currently, there are prospective studies for NSCLC and Renal Cell Carcinoma, such as the HALT and STOP-NSCLC trials, which are further investigating the clinical benefit of SBRT [Citation3].
Table 6. Oligoprogressive disease comparison summary.
When looking outside of NSCLC an 86 patient retrospective study on prostate cancer by Triggiani et al. [Citation26] found a median metastasis-free survival of 12.3 months with one- and two-year distant progression-free survival of 52.3 and 33.7%, respectively. Pembroke et al. [Citation27] studied 57 cases of oligoprogression in a variety of disease sites and found a median OS and PFS of 22 and 6.4 months, respectively. Cheung et al. performed a prospective phase II study of metastatic renal cell cancer OPD patients and found that the two-year OS was 77%, the CI of TS was 47% at 1 year and 75% at 2 years, with a median TS time of 12.6 months [Citation28]. The current study found a median OS of 25.1 months and a median PFS of 7.8 months and a CI of TS of 37.4% at 1 year and 43.9% at 2 years. which is consistent with the findings of these previous studies. This should also take into context the fact that there was a median time of diagnosis of oligoprogression of 24 months after their original metastatic diagnosis. This suggests that applying SBRT to progressive lesions can delay the need to start or switch systemic therapy and lead to a prolongation of PFS time.
Only five patients in this study had a local failure. Local control was 95% at 1 year and 92.7% at 2 years. Gan et al. [Citation29] found a local control of 86% at 12 months in NSCLC whereas Pembroke et al. found a local control of 48% at 12 months over various disease sites [Citation25]. Despite the variation between different studies, the experience at our center supports the idea that SBRT for this patient population will lead to effective local control. Unfortunately, it is worth noting that 56 patients in our study did progress distantly. Further work to elucidate the benefit that this treatment option has on quality of life and resolution of local symptoms would be beneficial in fully characterizing the benefit of SBRT in oligoprogressive disease.
With ongoing developments in systemic therapy and immunotherapy patients are living longer with widespread metastatic disease [Citation15,Citation24,Citation25]. By potentially prolonging the time before patients must switch or start new systemic therapy options SBRT for OPD patients could provide oncologists with additional time before moving on to next line systemic therapy. In this study thirty-eight patients (47%) changed systemic therapy following SBRT with a cumulative incidence of treatment switch of 43.9% at 2 years. Studies in various disease sites have demonstrated a variety of systemic or treatment-free survival timelines as seen in [Citation30–32]. In this study, the use of additional SBRT for 17 patients who had further OPD, despite operationally having an event that would classify as progression may have also contributed to the prolongation of time before the treatment switch. There is clearly variation in the literature between differing disease sites which is an important consideration for studies of OPD moving forward [Citation32]. This is likely caused by a multitude of factors including differences in the systemic therapy regimens that patients were on before their OPD and the inherent aggressiveness of various disease phenotypes.
In the current study, the multivariate analysis demonstrated that the time to oligoprogression was the only significant factor that influenced progression-free survival. Although not statistically significant the patients who had a time to oligoprogression under 24 months had a notably higher incidence of treatment switch at 1 year. This demonstrates that patients who quickly progressed to oligoprogressive disease after their initial malignancy diagnosis had a higher chance of requiring a treatment switch after SBRT. This difference between the two population groups could help to inform the design of future studies.
Although it is difficult to draw definitive conclusions on the effectiveness of SBRT to delay systemic therapy, with the advent of new treatment therapies and the increasing precision of radiation techniques there is an ongoing need to understand the benefits that this therapy can provide.
This study of course had several limitations. These include the retrospective nature of the analysis and the heterogeneity in disease site and patient characteristics. Additionally, this study design leads to inherent selection bias, including immortal time bias, given the long duration in general before administration of SBRT. Therefore, these patients may inherently have a better prognosis disease, and thus, respond more favorably to any therapy, including prolongation of systemic therapy without SBRT. Conversely, this could also indicate that clinicians (both referring and treating) are selecting patients carefully with the potentially better biological disease who may have more durable responses and future salvageable sites of progression. Moreover, changes in institutional policy, knowledge, and technology over time could have an impact on the findings. Overall though the study’s findings are consistent with the current literature and continue to suggest the potential benefit of SBRT for OPD, particularly in disease sites (breast, colorectal cancer) that may not be as well-studied as NSCLC and RCC. Further prospective studies are needed to further quantify the impact on PFS and OS as well as the implications of SBRT on the systemic therapy management of this patient population.
Conclusion
In summary, this was a retrospective institutional review aimed to investigate the impact of SBRT on patients with OPD. SBRT demonstrated the ability to effectively control locally progressing lesions without significant toxicities. However, while patients did still frequently experience distant recurrence, there was a considerable proportion of patients who had minimally progressing disease. Over half of the patients in this study did not require a treatment switch at two years. This can be realized through either continuation of a current systemic regimen or prolongation of their time of treatment. This could result in an improvement in patients’ quality of life or potentially prolong their PFS. Further prospective studies are warranted to validate and optimize the benefit of integrating SBRT with systemic therapy strategies to help improve the care of patients with OPD.
Disclosure statement
The authors report no conflicts of interest.
References
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13(1):8–10.
- Tumati V, Iyengar P. The current state of oligometastatic and oligoprogressive non-small cell lung cancer. J Thorac Dis. 2018;10(Suppl 21):S2537–S2544.
- Patel PH, Palma D, McDonald F, et al. The Dandelion dilemma revisited for oligoprogression: treat the whole lawn or weed selectively? Clin Oncol (R Coll Radiol). 2019;31(12):824–833.
- Cheung P. Stereotactic body radiotherapy for oligoprogressive cancer. Br J Radiol. 2016;89(1066):20160251.
- Kim C, Hoang CD, Kesarwala AH, et al. Role of local ablative therapy in patients with oligometastatic and oligoprogressive non-small cell lung cancer. J Thorac Oncol. 2017;12(2):179–193.
- Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing (vol 366, pg 883, 2012). N Engl J Med, 2012;367(10):976–976.
- Soucheray M, Capelletti M, Pulido I, et al. Intratumoral heterogeneity in EGFR-mutant NSCLC results in divergent resistance mechanisms in response to EGFR tyrosine kinase inhibition. Cancer Res. 2015;75(20):4372–4383.
- Campo M, Al-Halabi H, Khandekar M, et al. Integration of stereotactic body radiation therapy with tyrosine kinase inhibitors in stage IV oncogene-driven lung cancer. Oncologist. 2016;21(8):964–973.
- Kelly P, Ma Z, Baidas S, et al. Patterns of progression in metastatic estrogen receptor positive breast cancer: an argument for local therapy. Int J Breast Cancer. 2017;2017:1367159.
- Planchard D, Popat ST, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv192–iv237.
- Franceschini D, De Rose F, Cozzi S, et al. The use of radiation therapy for oligoprogressive/oligopersistent oncogene-driven non-small cell lung cancer: state of the art. Crit Rev Oncol Hematol. 2020;148:102894.
- Lara TM, Helou J, Poon I, et al. Multisite stereotactic body radiotherapy for metastatic non-small-cell lung cancer: delaying the need to start or change systemic therapy? Lung Cancer. 2018;124:219–226.
- Ettinger DS, Wood DE, Akerley W, et al. Non-small cell lung cancer, version 6.2015. J Natl Compr Canc Netw. 2015;13(5):515–524.
- Gridelli C, De Marinis F, Cappuzzo F, et al. Treatment of advanced non-small-cell lung cancer with epidermal growth factor receptor (EGFR) mutation or ALK gene rearrangement: results of an international expert panel meeting of the Italian Association of Thoracic Oncology. Clin Lung Cancer. 2014;15(3):173–181.
- Weickhardt AJ, Scheier B, Burke JM, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol. 2012;7(12):1807–1814.
- Triggiani L, Alongi F, Buglione M, et al. Efficacy of stereotactic body radiotherapy in oligorecurrent and in oligoprogressive prostate cancer: new evidence from a multicentric study. Br J Cancer. 2017;116(12):1520–1525.
- Santini D, Ratta R, Pantano F, et al. Outcome of oligoprogressing metastatic renal cell carcinoma patients treated with locoregional therapy: a multicenter retrospective analysis. Oncotarget. 2017;8(59):100708–100716.
- Benedict SH, Yenice KM, Followill D, et al. Stereotactic body radiation therapy: the report of AAPM task group 101. Med Phys. 2010;37(8):4078–4101.
- Sahgal A, Roberge D, Schellenberg D, et al. The Canadian Association of Radiation Oncology scope of practice guidelines for lung, liver and spine stereotactic body radiotherapy. Clin Oncol (R Coll Radiol). 2012;24(9):629–639.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247.
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the radiation therapy oncology group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31(5):1341–1346.
- Jewett MA, Zuniga A. Renal tumor natural history: the rationale and role for active surveillance. Urol Clin North Am. 2008;35(4):627–634.
- Saravana-Bawan B, Bajwa A, Paterson J, et al. Active surveillance of low-risk papillary thyroid cancer: a meta-analysis. Surgery. 2020;167(1):46–55.
- Xu Q, Liu H, Meng S, et al. First-line continual EGFR-TKI plus local ablative therapy demonstrated survival benefit in EGFR-mutant NSCLC patients with oligoprogressive disease. J Cancer. 2019;10(2):522–529.
- Borghetti P, Bonù ML, Giubbolini R, et al. Concomitant radiotherapy and TKI in metastatic EGFR- or ALK-mutated non-small cell lung cancer: a multicentric analysis on behalf of AIRO lung cancer study group. Radiol Med. 2019;124(7):662–670.
- Triggiani L, Mazzola R, Magrini SM, et al. Metastasis-directed stereotactic radiotherapy for oligoprogressive castration-resistant prostate cancer: a multicenter study. World J Urol. 2019;37(12):2631–2637.
- Pembroke CA, Fortin B, Kopek N. Comparison of survival and prognostic factors in patients treated with stereotactic body radiotherapy for oligometastases or oligoprogression. Radiother Oncol. 2018;127(3):493–500.
- Cheung P, Patel SI, North S, et al. Stereotactic radiotherapy for oligoprogression in metastatic kidney cancer patients receiving tyrosine kinase inhibitor therapy: a prospective phase II multi-centre study. Int J Radiat Oncol Biol Phys. 2020;108(3):S27–S28.
- Gan GN, Weickhardt AJ, Scheier B, et al. Stereotactic radiation therapy can safely and durably control sites of extra-Central nervous system oligoprogressive disease in anaplastic lymphoma kinase-positive lung cancer patients receiving crizotinib. Int J Radiat Oncol Biol Phys. 2014;88(4):892–898.
- Ingrosso G, Detti B, Fodor A, et al. Stereotactic ablative radiotherapy in castration-resistant prostate cancer patients with oligoprogression during androgen receptor-targeted therapy. Clin Transl Oncol. 2021;23(8):1577–1578.
- Ji X, Zhao Y, Zhu X, et al. Outcomes of stereotactic body radiotherapy for metastatic colorectal cancer with oligometastases, oligoprogression, or local control of dominant tumors. Front Oncol. 2021;10:3265.
- Meyer E, Pasquier D, Bernadou G, et al. Stereotactic radiation therapy in the strategy of treatment of metastatic renal cell carcinoma: a study of the Getug group. Eur J Cancer. 2018;98:38–47.
