The treatment of metastatic non–small cell lung cancer (NSCLC) has evolved rapidly in recent years. For patients with nonsquamous cell carcinoma with oncogene addiction, targeted therapies are the preferred treatment, whereas immunotherapy (IT) has revolutionized treatment options for those without oncogene addiction and those with squamous cell carcinoma. IT treatment options, with or without chemotherapy (CT) are based primarily on expression levels of programmed death ligand 1 (PD-L1) [Citation1]. These rapidly evolving treatment options and the molecular pathology testing required for optimal patient selection can be difficult to implement into daily practice, with technical (i.e., type of diagnostic test, expertise of the pathologist interpreting the results, and turnaround time) and reimbursement issues (i.e., treatment reimbursed without diagnostic test reimbursement) compromising PD-L1 testing. Real-world prescription data can serve as a tool for identifying such barriers to the implementation of optimal treatment. We therefore conducted a cross-sectional study to investigate the relationship between patient, tumor and treatment site characteristics, and systemic treatment choices for patients with untreated, stage IV NSCLC in the public health care system in Belgium with the aim of establishing a better understanding of the characteristics that impact real-life treatment decisions (NCT03959137; VEAP7678).
Consecutive patients with untreated stage IV NSCLC scheduled to receive systemic treatment or best supportive care (BSC) from June 2019 through October 2019 were included. Participants were aged ≥18 years with a histologically or cytologically confirmed diagnosis of stage IV NSCLC. The prospective collection of data started after a maximum of one cycle of treatment, except for patients receiving BSC only. Participants who had previously received systemic treatment for metastatic NSCLC were excluded; however, patients who had received earlier adjuvant or neoadjuvant therapy were eligible. Patients who had received a tyrosine kinase inhibitor, participated in a clinical trial, or received a novel therapy in a medical need program (i.e., patients who received systemic drugs free of charge outside of their usual prescription, based on reimbursement criteria) were also excluded.
A questionnaire was completed by the respiratory oncologist at each participating site regarding treatment site characteristics. Based on the average number of new NSCLC cases per year and on participation in clinical trials, sites were allocated into four categories of: high diagnostic volume (HDV; i.e., more than the median number of patients per year in that hospital) and participating/not participating in clinical trials; low diagnostic volume (LDV, less than the median number of patients) and participating/not participating in clinical trials. Additional site characteristics included capability to perform genetic and PD-L1 testing (for both in-house and referral). Treatment and patient characteristics were recorded in an electronic case report form, which included the category of the selected systemic treatment: CT, IT, IT-CT, or BSC. If indicated, palliative radiotherapy was given per standard clinical practice. Variables that positively or negatively impacted the choice of systemic treatment were documented, and physicians were also asked to identify the three most important variables that influenced their selection of treatment. Patient characteristics included in this assessment were demographics, medical history, comorbidities, presence of autoimmune disease, current or recent medications, and prior cancer treatment in earlier stage NSCLC (Supplementary Materials).
For this descriptive study, it was assumed that 200 patients would provide a representative picture of first-line systemic treatment decisions for stage IV NSCLC in Belgium. At most, 20 patients were permitted to be enrolled at a single site and at least 30 patients were required within each hospital category. Because of the issue of quasi-complete separation driven by the factor PD-L1 tumor proportion score (TPS), it was decided to perform subgroup analyses based on the PD-L1 score. Since for patients with high PD-L1 TPS ≥50%, the main interest was in the comparison of IT alone with IT-CT, the outcome variable was dichotomized. In both subgroups, simple logistic regression was used to initially identify important variables (p < .25) which were then explored through multiple logistic regression. Covariates considered for the simple models were age, sex, weight loss, smoking status, patient treatment preference, metastatic disease status, tumor diameter size (T-size), number of metastatic sites, brain metastases, liver metastases, concomitant malignancies, histology, comorbidities, autoimmune disease, use of corticosteroids/immunosuppressants, antibiotics, prior cancer treatment for local disease, and site type.
Across the 21 participating sites, the median number of newly diagnosed patients during 2018 was 143.7 (standard deviation, 68.5). Based on this median, 10 sites were classified as HDV centers (enrolling 116 patients) and 11 as LDV centers (enrolling 93 patients). Fifteen of the 21 (71.4%) sites were participating in clinical trials. Within the sites that were participating in clinical trials, genetic testing was not performed for 20.7% of patients enrolled at LDV sites compared with 10.6% of those at HDV sites.
A total of 209 patients were included (Supplementary Figure 1). The mean age was 68.2 years; 95.7% of patients were current or former smokers, 65.1% were male, 77% had an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 or 1, and 65.6% had nonsquamous histology (Supplementary Table 1). In the total population, 33% of patients had PD-L1 TPS <1% and 42.1% had PD-L1 TPS ≥50%. Patient characteristics were also generally similar regardless of diagnostic volume and clinical trial participation; however, the proportion of patients with PD-L1 TPS ≥50% was higher at HDV sites participating in clinical trials compared with LDV sites or those not participating in clinical trials (51.1% vs. 22.7–37.9%). This may be due to the possibility that patients with lower PD-L1 expression at these centers may have been participating in clinical trials and thus not included in the present study. This logically resulted in the inclusion of a higher proportion of patients with high PD-L1 TPS who were more likely to receive IT alone. LDV sites not participating in clinical trials did not report nonsmokers and had more squamous cell histology (42.9% vs. 18.2–34.5%). The proportion of patients with comorbidities was roughly twice as high at LDV centers compared with HDV centers, although age and smoking habits did not differ significantly. This may be due to physical and/or socioeconomic constraints that prevented these patients attending HDV centers. Overall, there was a relatively low incidence of autoimmune diseases (6.2%) and an even lower incidence of active autoimmune diseases (3.3%).
The six characteristics with the highest rate of impact on treatment decisions were PD-L1 TPS, ECOG PS, metastatic disease status, squamous vs. nonsquamous histology, age, and patient preference (). Characteristics with an ‘important’ impact rate of ≥10% for each systemic treatment choice are shown in Supplementary Table 2. For example, 94.1% of physicians indicated that PD-L1 TPS levels assumed a high rate of importance in their decision to prescribe IT. Similarly, PD-L1 TPS was assigned a high rate of importance by 76.8% of physicians prescribing IT-CT and 69.2% of those prescribing CT, whereas only 37.5% considered PD-L1 TPS to have a high rate of importance in the selection of BSC. Overall, PD-L1 expression was considered the most important factor in determining treatment, which seems logical, as it is the only objective factor in Belgium used to guide treatment reimbursement. Other important factors for >50% of physicians were ECOG PS for prescribing IT-CT (62.6%) or BSC (68.8%), the extent of metastatic disease when prescribing IT (54.4%), and patient preference when prescribing BSC (56.3%). Poor ECOG PS tended to guide physicians away from the use of IT-CT which may illustrate a fear of treatment-limiting toxicity in patients with poor ECOG PS and preference for treatment with the highest likelihood of success for fit patients. Poor ECOG PS also was an important factor in selecting BSC alone which again demonstrates the clinical selection of patients deemed fit or unfit for active treatment. Histology was an important factor for 42.4% of physicians in their consideration for IT-CT.
Figure 1. Factors with major impact on treatment choice. BSC: best supportive care; CT: chemotherapy; IT: immunotherapy; IT-CT: immunotherapy + chemotherapy; PD-L1: programmed death ligand 1; ECOG PS: Eastern Cooperative Oncology Group performance status; TPS: tumor proportion score.
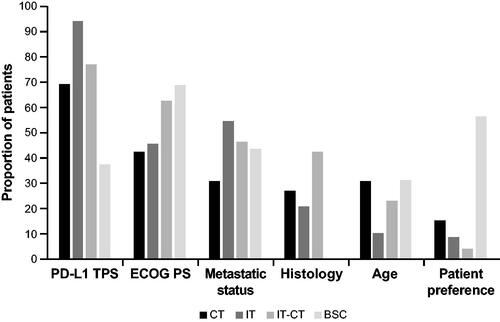
The most common treatment was IT-CT (47.4%), followed by IT alone (32.5%), CT (12.4%), and BSC (7.7%). The high proportion of patients who received IT, either alone or in combination with CT, reflects a high adherence to international treatment guidelines and a similar access to standard-of-care treatment options between hospitals, regardless of their diagnostic volume or participation in clinical trials. Choices of systemic treatment stratified by key patient characteristics are shown in Supplementary Table 3. Most patients with PD-L1 TPS <50% received IT-CT (73.9% in PD-L1 < 1%/73.3% in PD-L1 1–49%), whereas 76.1% of those with PD-L1 TPS ≥50% received IT alone. Almost half of all patients with ECOG PS 2 (47.1%) received IT, whereas 63.6% of those with ECOG PS 3/4 received BSC. Never-smokers were rare, but IT was selected for only one of eight (12.5%), compared with 32.5% for the total study population.
Logistic regression was conducted to examine treatment choices in patients with PD-L1 ≥ 50% and PD-L1 < 50%. Eight patients were not included because their treatment categories were underrepresented (see Supplementary Materials). Within the group of patients with PD-L1 TPS <50%, simple logistic regression identified age, weight loss (≤5% vs. >5%), smoking status, ECOG PS, patient preference, and potentially antibiotics as significantly associated with treatment choice which were further assessed using multiple logistic regression (). Age (p = .0308) and ECOG PS (p = .0005) had a significant impact on the treatment selection, whereas patient preference was borderline significant (p = .0585). The probability of receiving CT or BSC versus IT-CT increased with age (odds ratio [OR] 1.090; 95% CI 1.005–1.182 and OR 1.148; 95% CI 1.004–1.313). The model also indicated a lower probability of receiving CT or BSC compared with IT-CT in patients with lower ECOG PS (OR 0.084; 95% CI 0.016–0.450 and OR 0.008; 95% CI <0.001–0.113). Finally, the probability of receiving BSC was lower if patient preference was not BSC or unknown compared with patients who expressed a preference for BSC (OR 0.010; 95% CI <0.001–0.354 and OR 0.029; 95% CI 0.002–0.533).
Figure 2. Multiple regression results for patients with PD-L1 TPS ≥50% and <50%. BSC: best supportive care; CT: chemotherapy; ECOG PS: Eastern Cooperative Oncology Group performance status; IT: immunotherapy; IT-CT: immunotherapy + chemotherapy; PD-L1 TPS: programmed death ligand 1 tumor proportion score.
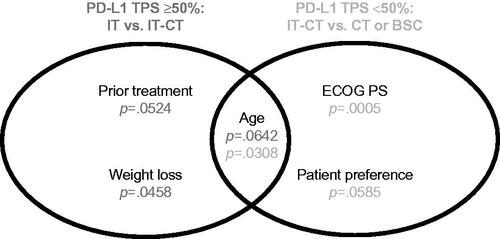
Within the group of patients with PD-L1 TPS ≥50%, age, weight loss, tumor size diameter, comorbidities, and prior cancer treatment were potentially associated with treatment choice; however, there were no variables that significantly impacted treatment selection (age, weight loss, and prior cancer therapy had a borderline significant impact). Ultimately, given the limited sample size in this study, it remains difficult to definitively state which of the factors that were significant according to the univariate analysis are clearly decisive for each PD-L1 category.
This observational study provided detailed information about patient characteristics and factors impacting treatment decision in patients with treatment-naive stage IV NSCLC from Belgium. The strengths of this study are: (1) the national coverage providing a realistic picture of daily oncologic care in Belgium, (2) the well-balanced enrollment between HDV and LDV centers, (3) the data collection period that encompasses the recent changes in treatment guidelines with the availability of IT, and (4) the small proportion of missing data. The limitations of this study include (1) the absence of some treatment options (i.e., IT-CT + bevacizumab and dual IT) because of local reimbursement policies and the exclusion of patients receiving treatment through a medical need program; (2) the limited sample size, which did not permit the optimal representation of specific populations (e.g., patients with autoimmune diseases).
In conclusion, our study confirms the adherence of Belgian thoracic oncologists to current guidelines with the large-scale implementation of PD-L1 testing and IT as the first-line treatment for advanced, non–oncogenic driven NSCLC. PD-L1 expression level and ECOG PS were shown to be major determinants in the choice of treatment. Finally, physicians use additional selection criteria, such as age, comorbidities, weight loss, and extent of metastatic disease, when selecting the best treatment options for their patients.
Supplemental Material
Download MS Word (97.8 KB)Acknowledgements
The authors thanks to Hermine Leroi for the operational support and assistance during this project. The authors thank Katrien Willaert for the initiation of the study and its study design.
Disclosure statement
A. Sibille received support for the present study from Merck Sharp & Dohme. F. Bustin has received funding from Roche. L. Carestia, Lynn Decoster, S. Derijcke, S. Ocak, C. Oyen, K. Pat, and V. Pruniau have nothing to disclose. G. Catala has served on an advisory board and received travel support for congress attendance from Bristol Myers Squibb. C. Compere has received consulting fees from Roche; payments/honoraria from Pfizer; travel support for meeting attendance from Roche, Merck Sharp & Dohme, and Pfizer; and has served on a data safety monitoring board or advisory board for AstraZeneca and Roche. K. Cuppens has received consultancy fees from Merck Sharp & Dohme, Johnson & Johnson, Roche, Bristol Myers Squibb, and AstraZeneca; has received travel support for meeting attendance from Roche; and has served on a data safety monitoring board or advisory board for PDC Pharma, Merck Sharp & Dohme, Roche, Bristol Myers Squibb, Boehringer Ingelheim, and AstraZeneca. B. Colinet has received consulting fees from Boehringer Ingelheim, Bristol Myers Squibb, Merck Sharp & Dohme, Bayer, Eli Lilly, AstraZeneca, and Amgen; and has received royalty or license payments from SRL BC Pneumologue. S. Coulon and S. Vandekeere are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and own stock in Merck & Co., Inc., Kenilworth, NJ, USA. N. De Brucker has received support for meeting attendance from Chiesi and Merck Sharp & Dohme and has received research grants from Roche. L. Decoster has received fees, payments, or honoraria from Merck Sharp & Dohme; research grants from Boehringer Ingelheim; travel support for meeting attendance from AstraZeneca, Merck Sharp & Dohme, and Roche; and has served on a data safety monitoring board or advisory board for Bristol Myers Squibb, Lilly, Roche, Merck Sharp & Dohme, and AstraZeneca. I. Demedts has received consultancy fees from Merck Sharp & Dohme and payments or honoraria from AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Merck Sharp & Dohme, and Roche. K. Deschepper has received funding from Bristol Myers Squibb, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, and AstraZeneca and has received consulting fees from AbbVie, AstraZeneca, and Bristol Myers Squibb. D. Galdermans has received support from Merck Sharp & Dohme for the present study; has served on advisory boards for AstraZeneca, Bristol Myers Squibb, Merck Sharp & Dohme, and Boehringer Ingelheim; and has served leadership or fiduciary roles for AstraZeneca and Boehringer Ingelheim. A. Janssens has received nonfinancial support from Boehringer Ingelheim for congress attendance. T. Peters has received payments or honoraria and has served on a data safety monitoring board or advisory board for Merck Sharp & Dohme. V. Surmont has received payments or honoraria for meeting attendance and has served on a data safety monitoring board or advisory board for AstraZeneca and Roche. J. Vansteenkiste has received funding for this study from Merck Sharp & Dohme; has received scientific grants from Merck Sharp & Dohme; has received payments or honoraria from AstraZeneca, Bristol Myers Squibb, Eli Lilly, Janssen, Merck Sharp & Dohme, and Novartis; and has served on an advisory board for AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Janssen, Daiichi Sankyo, Merck Sharp & Dohme, Novartis, Pfizer, and Roche.
Data availability statement
Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA (MSD) is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company’s clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the US and EU or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
Reference
- Planchard D, Popat S, Kerr K, ESMO Guidelines Committee, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv192–iv237.
