Abstract
Background
Brain metastasis (BM) are uncommon among women with epithelial ovarian cancer (EOC). The frequency, risk factors and clinical repercussions of BM in these patients are not well described.
Methods
We retrospectively evaluated EOC patients treated at our center from 2002 to 2020 and assessed their clinical parameters, risk for BM development and association with overall survival (OS). This cohort has a known high frequency of BRCA mutation carriers (BRCAm) due to women of Ashkenazi Jewish descent.
Results
Among 1035 EOC patients, 29 (2.8%) were diagnosed with BM. The prevalence of BRCA mutations was more common among women with BM (56.5% vs. 34.3%, p = 0.033). The BM rate in patients with BRCAm was higher than the BM rate in those with wildtype BRCA (BRCAw; 5.1% vs. 2.1%, OR = 2.6; 95% CI: 1.2–5.4, p = 0.013). Median time from diagnosis to BM and from disease recurrence to BM was longer among patients with BRCAm. Median OS was not significantly different among patients with BM versus those without BM (59.4 vs. 73.4 months, p = 0.243). After BM diagnosis, median OS was not statistically significantly different between patients with BRCAm and those with BRCAw (20.6 vs. 12.3 months, p = 0.441). Treatment with poly (ADP-ribose) polymerase inhibitors and bevacizumab had no impact on subsequent development of BM.
Conclusions
BM are rare among EOC patients. However, the risk is three-fold higher among patients with BRCAm. BM do not significantly alter OS among EOC patients. The higher rate of BM in patients with BRCAm may be related to longer OS in this subpopulation.
Introduction
Brain metastases (BM) are a common complication of cancer and have been reported in up to 40% of patients with varying prevalence across malignancies and histology [Citation1–5]. The clinical presentation of patients with BM varies widely. Increased screening and improvements in imaging technologies have increased the percentage of patients that are diagnosed with asymptomatic disease [Citation6]. Symptoms associated with BM include intracranial hypertension, headache, seizures and/or focal neurological symptoms [Citation7]. Most (>90%) patients with BM have some decline in neurocognitive function prior to whole brain radiotherapy (WBRT) [Citation8,Citation9].
BM incidence and prognosis are related to tumor subtype [Citation10], the size and invasiveness of the metastasis, the number of BM, absence of systemic metastases, primary tumor control, age and key performance status [Citation7]. Prognosis is also affected by molecular features such as human epidermal growth factor receptor 2 (HER2) overexpression in breast cancer [Citation11,Citation12] and gastric cancer [Citation13], or epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) mutations in non-small cell lung cancer [Citation14,Citation15].
Historically, the outcome of patients with BM was poor but advances in imaging, surgery, radiotherapy, and medical oncology have allowed for earlier detection, optimal local treatment strategies mitigating potential complications, improving the survival and quality of life of these patients.
Surgery and radiotherapy are the mainstay of BM treatment. Surgery is mostly recommended for patients when there is a need to establish histologic diagnosis or for large lesions causing mass effect [Citation16,Citation17]. Radiotherapy can be delivered as WBRT or stereotactic radiosurgery (SRS). WBRT may cause late post-radiation cognitive decline [Citation18,Citation19] and SRS can be complicated by radionecrosis [Citation20]. BM can induce peritumoral edema, and corticosteroids may temporarily control brain edema. Although seizures may be the presenting symptom in up to 25% of BM, no immediate or long-term benefit from prophylactic use of antiepileptic drugs was found in patients without a previous history of seizures [Citation21–23].
Ovarian cancer (OC) is the fifth leading cause of cancer mortality in women, with approximately 21,000 new cases in the United States each year [Citation24]. Patients with pathogenic variants of BRCA1 and BRCA2 have increased risk of OC [Citation25,Citation26]. Approximately 18% of women with OC harbor BRCA1 or BRCA 2 mutations [Citation27,Citation28], with a higher prevalence among Ashkenazi Jewish women [Citation29]. One in 40 Ashkenazi Jewish women is a carrier of a BRCA mutation (BRCAm) [Citation30]. BRCA1 mutation carriers have an estimated 40–50% risk of developing OC by age 70, compared to 10–20% with BRCA2 mutations [Citation31].
Poly (ADP-ribose) polymerase (PARP) inhibitors propagate single-strand DNA breaks and lead to the accumulation of double stranded breaks, requiring repair by homologous recombination mechanisms. Patients with BRCAm respond to PARP inhibitors and have improved survival [Citation32,Citation33]. The brain is often regarded as a sanctuary site, with radiotherapy as the primary treatment modality for BM due to the low penetrance of systemic therapies across the blood-brain barrier, but some studies have considered aggressive treatments more effective, including chemotherapy [Citation34,Citation35]. Some works have suggested that PARP inhibitors, which are widely integrated into the management of women with BRCAm, homologous recombination deficiency (HRD) and recurrent disease, may have anti-neoplastic activity in the brain [Citation36–39]. Bevacizumab, commonly administered to women with stage-IV OC and residual disease, has demonstrated efficacy in BM among other primary malignancies [Citation40]. The effectiveness of PARP inhibitors and bevacizumab has yet to be investigated in OC patients with BM.
To date, the frequency of BM has not been extensively reported among patients with OC. The literature suggests an incidence ranging from 0.3% to 4.6% [Citation41–48], with similar incidence among patients with advanced serous OC and primary peritoneal carcinoma [Citation42]. In a large cohort of 4515 patients with OC, women with BRCAm had an increased four-fold risk of BM compared to women with wildtype BRCA (BRCAw); 3% vs. 0.6%) [Citation44]. BRCAm are more common among breast cancer patients with BM [Citation39,Citation49].
The aim of this retrospective study was to describe the incidence and characteristics of OC patients with BM. We assessed risk factors associated with BM development, the implication of BRCA status on outcomes of the disease, treatment with PARP inhibitors and bevacizumab, and prognostic factors influencing survival.
Methods
Study population
Medical records of 1035 consecutive patients with epithelial ovarian cancer (EOC), tubal carcinoma (TC) or primary peritoneal carcinoma (PPC), treated at the Oncology Division of Sourasky Tel Aviv Medical Center from January 2002 to May 2021, were retrospectively reviewed. Inclusion criteria included females with histopathologically diagnosed ovarian, tubal or primary peritoneal cancer (>18 years old).
The local Institutional Review Board (IRB) approved the study.
Collected data included demographic and clinical characteristics, systemic therapies, brain radiotherapy procedures, survival status, molecular profiles and BRCAm status.
BRCA germline is widely tested at our institute since 2003. Next generation sequencing has been used since 2010 but it is not always reimbursed, and HRD tests have mainly been used since 2020 (i.e. in the last year included in this analysis).
Outcomes
Overall survival (OS) was defined as the time from OC diagnosis to death or last follow-up. OS after BM diagnosis was defined as the time from BM diagnosis to death. Progression-free survival (PFS) was defined as time from the end of first platinum-based therapy to progression or recurrence or last follow-up. Platinum-sensitive ovarian cancer was defined as an interval greater than 6 months between the last cycle of platinum-based chemotherapy and the start of the subsequent course of platinum upon relapse.
Treatment issues
Radiosurgery or fractionated stereotactic radiotherapy was usually used to treat patients with up to 3 BM and a good performance status. Patients with multiple BM, leptomeningeal spread and poor performance status were treated with WBRT.
Concerning OC, women with systemic disease also received chemotherapy, while patients who had BM without evidence of systemic disease were followed-up.
Statistical analysis
Statistical analysis was performed using R version 4.0.5 (R Development Core Team, Vienna, Austria). Chi-squared tests and t-tests were used as appropriate to determine the differences between clinical characteristics of patients with BM and patients with no BM. OS and PFS were analyzed using Kaplan-Meier plots with log rank tests for differences between groups. Multivariate cox regression analysis was performed to evaluate PFS and OS while adjusting for possible confounders. Two-tailed p values <0.05 were considered statistically significant.
Results
Patient characteristics
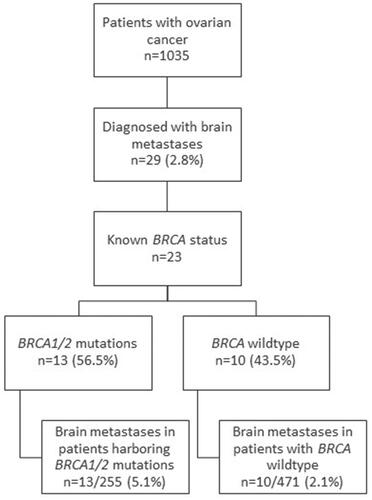
Of 1035 consecutive OC patients with a histopathological confirmation of OC, 29 women (2.8%) were diagnosed with BM. The clinical characteristics of the study population are presented in . The median age at diagnosis was 59.8 years in women with BM and 61.2 years in those without BM (p = 0.543). Approximately half of the women were of Ashkenazi Jewish descent in both groups. The majority of patients with BM were initially diagnosed with a more advanced disease, stage III-IV compared to 84.31% of women with no BM. The odds ratio (OR) for BM development with stage III compared to stages I–II was 1.96 (95% confidence interval [CI], 0.5–8.6), p = 0.370 and 4.78 for stage IV compared to stages I–II (95% CI, 1.03–22.2), p = 0.046. The histological distribution of OC was similar in women with BM and in those with no BM with serous papillary being the most prevalent in both groups. Platinum sensitivity was not statistically significantly different among those with and without BM. Three patients with BM (10.4%) had a history of breast cancer and another patient (3.5%) had synchronous melanoma with OC. Two patients (6.9%) had BM at OC diagnosis and 7 patients (24.1%) had their first metastasis at another site other than the brain. The rest (20/29, 69%) only had BM.
Table 1. Demographics and clinical parameters of ovarian cancer patients.
BM and BRCA carrier status
Of 1006 OC patients with no BM, 703 (67.9%) were tested for BRCA. Two-thirds (65.6%) were negative for BRCAm. Among the 242 women (34.4%) harboring a germline or somatic BRCAm 69.8% and 30.2% were BRCA1 and BRCA2 mutation carriers, respectively. Of 23/29 (79.3%) patients with BM who were screened for BRCAm, 56.5% (13/23)had BRCAm: 84.6% (11/13) harbored a BRCA1 mutation and 15.4% (2/13) harbored a BRCA2 mutation. The prevalence of BRCAm was more common among women with BM (56.5% vs. 34.4%, p = 0.033). The rate of BM in patients with BRCAm was higher than the rate of BM in patients with BRCAw (5.1% vs. 2.1%, p = 0.013), with a OR of 2.6 (95% CI, 1.2–5.4), ().
BM characteristics and treatments
Two patients (6.9%) had BM at OC diagnosis and seven patients (24.1%) had their first metastasis at another site other than the brain. The rest (20/29, 69%) only had BM.
Among the patients diagnosed with BM, most (17/29, 58.6%) presented with limited central nervous system (CNS) disease, involving 1–2 lesions. Four patients presented with leptomeningeal disease (LMD) and two had more than ten metastases. All patients had symptoms at BM diagnosis. The most common symptoms were a new neurological deficit, headaches and seizures.
Twelve patients (41.4%) underwent resection of the brain lesion. Sixteen patients (55.2%), including all four patients with LMD, were treated with WBRT, and the rest received focal radiotherapy (radiosurgery or fractionated stereotactic radiotherapy).
Ten patients (34.5%) had documented CNS disease progression and 14 (48.3%) women did not have progressive CNS disease. Five patients did not have response data. None of the four patients with LMD completed the planned course of WBRT: one patient died during the radiotherapy course and the other three died within a month from radiotherapy.
A greater percentage of patients with BM received PARP inhibitors compared to those with no BM. In the BM group, four women received PARP inhibitors prior to BM diagnosis, and five after BM diagnosis.
Bevacizumab was administered to 24.5% of patients with no BM and to 34.5%. Nine patients (90%) received bevacizumab before BM diagnosis, one patient (10%) received bevacizumab following BM diagnosis.
Time to BM development and OS
As shown in , the median time from OC diagnosis to BM diagnosis was 31.0 months (range 3.2–143.5). Patients with BRCAm were diagnosed with BM later than those with BRCAw (median, 44.3 months vs. 26.4 months, p = 0.044). Women with BRCAm also had longer median time from disease recurrence to BM development compared to those with BRCAw, but this difference was not statistically significant (median, 11.8 months vs. 2.7 months, p = 0.137). No statistically significant difference in median OS was found between patients with BRCAm whether or not they had BM (111.4 months vs. 105.5 months, p = 0.389).
Table 2. Comparison of OS by BM among the study population.
Patients with BM had a similar 3-year survival rate to those with no BM (79.3% vs. 80.7%, p = 1.00). Median OS was 59.4 months for patients with BM and 73.4 months for patients with no BM (p = 0.243, ).
Figure 2. Overall survival of patients with ovarian cancer by brain metastases diagnosis. Kaplan–Meier survival analysis showing overall survival by brain metastases diagnosis in patients with ovarian cancer. p = 0.243. BM: Brain metastases.
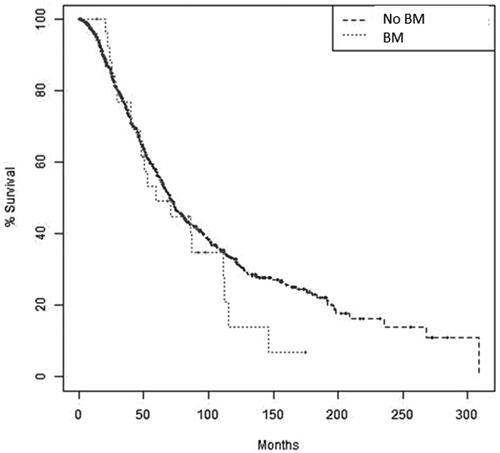
Figure 3. Overall survival of ovarian cancer patients with BM by BRCA mutation status. Kaplan–Meier survival analysis showing overall survival in patients with ovarian cancer and BRCA wildtype that were diagnosed with brain metastases compared to patients with ovarian cancer harboring BRCA1/2 mutations that were diagnosed with brain metastases. p = 0.441.
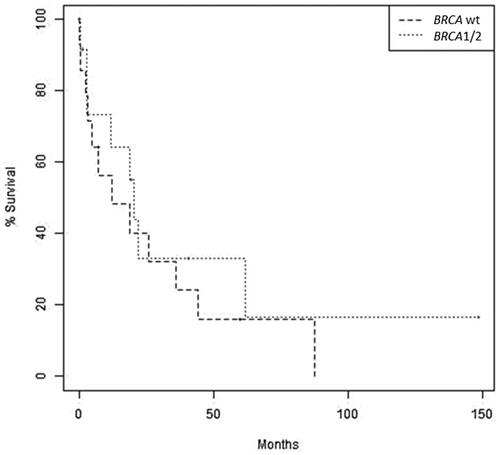
Median OS after BM diagnosis was 19.5 months. Median OS was longer in patients with a BRCAm compared to those with BRCAw, but not statistically significantly different (20.6 months vs. 12.3 months, p = 0.441, ). Analysis by BRCA subtype showed that women harboring a BRCA1 mutation had a longer median OS of 22.1 months (95% CI, 7.4–NE) compared to those harboring a BRCA2 mutation (9.3 months [95%CI, 19.0–NE]) and those with BRCAw (12.3 months [95%CI, 7.4–NE]) with a trend for a statistically significant difference among these populations (p = 0.073).
Discussion
We report on one of the largest cohorts to date of patients with OC and BM, including an extensive representation of patients with BRCAm. The overall prevalence of BM in this cohort of 1035 women with OC is low (2.8%), reaffirming previous reports (0.3–4.6%) [Citation41–44]. The prevalence of BM was almost 3-fold higher in BRCAm carriers compared to those with BRCAw (5.1% vs. 2.1%, p = 0.013). This finding is similar to other works describing BM in women with OC [Citation44,Citation45], and also reflects the relatively high rate of BRCAm in women with breast cancer who also have BM [Citation39,Citation49]. The higher prevalence of BM in patients with BRCAm might be attributed to the longer survival of this population because BM develop at a later stage in the disease course of patients with BRCAm (median time to BM development was 44.3 months compared to 26.4 months, respectively, p = 0.044). Another hypothesis requiring further investigation is the possible higher brain tropism among patients carrying BRCAm.
The non-statistically significant OS rate observed among EOC patients with and without BM suggests that BM are not associated with early mortality, although they may lead to neurological decline and affect patients’ quality of life. The unaltered OS rate may be attributed to patients’ limited brain disease (58.6% had 1–2 BM) and to an aggressive treatment approach. Approximately 40% of patients underwent surgery and all patients were treated with radiotherapy. Keskin et al. reported that longer time from initial diagnosis to BM, treatment with radiotherapy, and optimal cytoreductive operation were positively correlated with longer OS of EOC patients with BM [Citation50]. Nasu et al. have found that ovarian/tubal/peritoneal origin, a Karnofsky performance status above 70, a single BM, absence of extracranial disease, cranial surgery, cranial radiotherapy, and chemotherapy are independent favorable prognostic factors for survival [Citation51]. Survival after diagnosis of BM is also affected by primary disease status (controlled versus uncontrolled) and extent (cranial metastases only versus cranial and extracranial metastases) and by the number, volume, and site of metastases in the brain parenchyma [Citation46,Citation48]. treatment-free interval of <6 months and no anti-cancer treatment for BM were also reported to be independently related to poor survival [Citation46].
In our cohort, the median OS after diagnosis of BM (19.5 months) was also longer than expected. Piura and Piura [Citation48] reviewed 36 series comprising a total of 513 patients and found that the median survival after diagnosis of BM was 6.4 months (range 1–28 months); most of the reviewed studies reported median survival periods of less than 12 months. Similarly, Keskin et al. [Citation50] reported median survival of 9 months after the initial diagnosis of BM in a cohort of 21 patients with EOC, and Bahat et al. [Citation52] reported median survival time of 4.5 months after BM diagnosis in a cohort of 10 patients with OC. Similar to Stasenko et al. [Citation43], our results showed that patients with a BRCAm had a non-statistically significant longer survival time following BM diagnosis compared to patients with BRCAw. The addition of radiotherapy to surgery has been shown to decrease the risk for neurological death as well as to decrease local recurrence and tumor recurrence anywhere in the brain but it did not affect survival [Citation53–56]. The strength of our institution’s approach is in the aggressive management of BM using surgery whenever possible, in this case in 40% of patients. We believe that this approach provides symptomatic and disease control benefit. A similar approach was previously reported to be beneficial in other trials [Citation48,Citation49]. Additionally, our cohort had a relatively high number of BRCAm patients with better median OS.
Approximately 30% of OC patients with BM were treated with PARP inhibitors and/or bevacizumab, which are believed to have higher penetration of the blood brain barrier than traditional chemotherapy [Citation36–40]. However, these systemic therapies did not influence the incidence of BM or OS following the diagnosis of BM. The lack of therapeutic impact upon the brain is emphasized by the higher proportion of patients treated with PARP inhibitors and bevacizumab in the BM group compared to patients with no BM (∼30% vs. ∼10–20%). Direct evaluation of brain tumor response to PARP inhibitors and bevacizumab in needed to better understand their direct role in this clinical setting. This study provides initial insight, suggesting their minimal contribution in mitigating the development of BM.
The strength of the study lies in the use of real-life data of a relatively large cohort treated in an onco-gynecological unit over a period of 18 years. The study limitations include its retrospective nature. In addition, it is likely that the study cohort reflects a founder population of Ashkenazi Jewish women with a distinct distribution of BRCAm (35.1%). Additionally, the BRCA status was tested in a higher number of patients with BM compared to patients with no BM (79.3% vs. 67.9%), possibly influencing the results. A few other markers, such as HRD, are known to have prognostic value in OC [Citation57,Citation58]. However, only a small number of patients were tested for HRD, as it has been integrated into routine practice only recently (in 2020), potentially limiting the population of patients eligible for PARP inhibitors. Another important limitation is the small number of patients with OC and BM, restricting our ability to perform subgroup analysis.
In conclusion, although BM are infrequent among OC patients, the risk for BM is almost three-fold higher among patients with BRCAm. Interestingly, BM did not significantly alter the OS survival of OC patients, and their OS was longer than that reported for other malignancies with BM. Our work suggests that the higher rate of BM in patients with BRCAm may be related to their longer survival. The use of newer systemic therapies does not seem to alter the incidence of BM in this patient population or to affect patient OS, but this should be evaluated in a larger cohort of OC patients with BM. We suggest that clinicians should lower the threshold for brain imaging in OC patients with BRCAm. Further trials to evaluate other risk factors for BM in OC and the direct effect of systemic therapies are warranted.
Supplemental Material
Download PDF (117.8 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data is not available.
References
- Barnholtz-Sloan JS, Sloan AE, Davis FG, et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the metropolitan Detroit cancer surveillance system. J Clin Oncol. 2004;22(14):2865–2872.
- Schouten LJ, Rutten J, Huveneers HA, et al. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, Colon, kidney, and lung and melanoma. Cancer. 2002;94(10):2698–2705.
- Cagney DN, Martin AM, Catalano PJ, et al. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: a population-based study. Neuro Oncol. 2017;19(11):1511–1521.
- Habbous S, Forster K, Darling G, et al. Incidence and real-world burden of brain metastases from solid tumors and hematologic malignancies in Ontario: a population-based study. Neurooncol Adv. 2021;3(1):vdaa178.
- Ali S, Gorska Z, Duchnowska R, et al. Molecular profiles of brain metastases: a focus on heterogeneity. Cancers. 2021;13(11):2645.
- Steindl A, Brunner TJ, Heimbach K, et al. Changing characteristics, treatment approaches and survival of patients with brain metastasis: data from six thousand and thirty-one individuals over an observation period of 30 years. Eur J Cancer. 2022;162:170–181.
- Bertolini F, Spallanzani A, Fontana A, et al. Brain metastases: an overview. CNS Oncol. 2015;4(1):37–46.
- Mehta MP, Rodrigus P, Terhaard CH, et al. Survival and neurologic outcomes in a randomized trial of motexafin gadolinium and whole-brain radiation therapy in brain metastases. J Clin Oncol. 2003;21(13):2529–2536.
- Meyers CA, Smith JA, Bezjak A, et al. Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: results of a randomized phase III trial. J Clin Oncol. 2004;22(1):157–165.
- Sperduto PW, Mesko S, Li J, et al. Survival in patients with brain metastases: summary report on the updated diagnosis-specific graded prognostic assessment and definition of the eligibility quotient. J Clin Oncol. 2020;38(32):3773–3784.
- Kennecke H, Yerushalmi R, Woods R, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28(20):3271–3277.
- Martin AM, Cagney DN, Catalano PJ, et al. Brain metastases in newly diagnosed breast cancer: a population-based study. JAMA Oncol. 2017;3(8):1069–1077.
- Limon D, Gal O, Gordon N, et al. Brain metastasis in gastroesophageal adenocarcinoma and HER2 status. J Neurooncol. 2018;138(2):315–320.
- Iuchi T, Shingyoji M, Itakura M, et al. Frequency of brain metastases in non-small-cell lung cancer, and their association with epidermal growth factor receptor mutations. Int J Clin Oncol. 2015;20(4):674–679.
- Rangachari D, Yamaguchi N, VanderLaan PA, et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer. 2015;88(1):108–111.
- Nabors LB, Portnow J, Ahluwalia M, et al. Central nervous system cancers, version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020;18(11):1537–1570.
- Tsao MN, Rades D, Wirth A, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): an American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2012;2(3):210–225.
- Tallet AV, Azria D, Barlesi F, et al. Neurocognitive function impairment after whole brain radiotherapy for brain metastases: actual assessment. Radiat Oncol. 2012;7:77.
- Brown P, Asher A, Ballman K, et al. BMET-05: NCCTG N0574 (ALLIANCE): a phase III randomized trial of whole brain radiation therapy (WBRT) in addition to radiosurgery (SRS) in patients with 1 to 3 brain metastases. Neuro Oncol. 2015;17(Suppl 5):v45–v46.
- Tsao M, Xu W, Sahgal A. A Meta-analysis evaluating stereotactic radiosurgery, whole-brain radiotherapy, or both for patients presenting with a limited number of brain metastases. Cancer. 2012;118(9):2486–2493.
- Mikkelsen T, Paleologos NA, Robinson PD, et al. The role of prophylactic anticonvulsants in the management of brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):97–102.
- Sirven JI, Wingerchuk DM, Drazkowski JF, et al. Seizure prophylaxis in patients with brain tumors: a meta-analysis. Mayo Clin Proc. 2004;79(12):1489–1494.
- Tremont-Lukats IW, Ratilal BO, Armstrong T, et al. Antiepileptic drugs for preventing seizures in people with brain tumors. Cochrane Database Syst Rev. 2008;16(2):CD004424.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA A Cancer J Clin. 2021;71(1):7–33.
- Miki Y, Swensen J, Shattuck-Eidens D, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266(5182):66–71.
- Wooster R, Neuhausen SL, Mangion J, et al. Localization of a breast cancer susceptibility gene, BRCA2, to chromosome 13q12-13. Science. 1994;265(5181):2088–2090.
- Walsh CS. Two decades beyond BRCA1/2: homologous recombination, hereditary cancer risk and a target for ovarian cancer therapy. Gynecol Oncol. 2015;137(2):343–350.
- Walsh T, Casadei S, Lee MK, et al. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc Natl Acad Sci U S A. 2011;108(44):18032–18037.
- Metcalfe KA, Poll A, Royer R, et al. Screening for founder mutations in BRCA1 and BRCA2 in unselected Jewish women. J Clin Oncol. 2010;28(3):387–391.
- Casanova DR. Beckmann and ling’s obstetrics and gynecology. 8th ed. Philadelphia (PA): LWW; 2018.
- Webb PM, Jordan SJ. Epidemiology of epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol. 2017;41:3–14.
- Xu K, Yang S, Zhao Y. Prognostic significance of BRCA mutations in ovarian cancer: an updated systematic review with meta-analysis. Oncotarget. 2017;8(1):285–302.
- Haunschild CE, Tewari KS. The current landscape of molecular profiling in the treatment of epithelial ovarian cancer. Gynecol Oncol. 2021;160(1):333–345.
- Geisler JP, Geisler HE. Brain metastases in epithelial ovarian carcinoma. Gynecol Oncol. 1995;57(2):246–249.
- Anupol N, Ghamande S, Odunsi K, et al. Evaluation of prognostic factors and treatment modalities in ovarian cancer patients with brain metastases. Gynecol Oncol. 2002;85(3):487–492.
- Gray S, Khor XY, Yiannakis D. Niraparib as maintenance therapy in a patient with ovarian cancer and brain metastases. BMJ Case Rep. 2019;12(8):e230738.
- O'Sullivan CC, Davarpanah NN, Abraham J, et al. Current challenges in the management of breast cancer brain metastases. Semin Oncol. 2017;44(2):85–100.
- Tao M, Cheng J, Wu X. Niraparib as maintenance therapy in germline ATM-mutated and somatic BRCA2-mutated ovarian cancer with brain metastases: a case report and literature review. Onco Targets Ther. 2020;13:12979–12986.
- Diossy M, Reiniger L, Sztupinszki Z, et al. Breast cancer brain metastases show increased levels of genomic aberration-based homologous recombination deficiency scores relative to their corresponding primary tumors. Ann Oncol. 2018;29(9):1948–1954.
- Mansouri A, Padmanaban V, Aregawi D, et al. VEGF and immune checkpoint inhibition for prevention of brain metastases: systematic review and meta-analysis. Neurology. 2021;97(15):e1484–e1492.
- Borella F, Bertero L, Morrone A, et al. Brain metastases from ovarian cancer: current evidence in diagnosis, treatment, and prognosis. Cancers. 2020;12(8):2156.
- Li X, Yang Q, Chen M, et al. Differences between primary peritoneal serous carcinoma and advanced serous ovarian carcinoma: a study based on the SEER database. J Ovarian Res. 2021;14(1):40.
- Stasenko M, Cybulska P, Feit N, et al. Brain metastasis in epithelial ovarian cancer by BRCA1/2 mutation status. Gynecol Oncol. 2019;154(1):144–149.
- Ratner E, Bala M, Louie-Gao M, et al. Increased risk of brain metastases in ovarian cancer patients with BRCA mutations. Gynecol Oncol. 2019;153(3):568–573.
- Jernigan AM, Mahdi H, Rose PG. Epithelial ovarian cancer metastatic to the central nervous system and a family history concerning for hereditary breast and ovarian cancer-a potential relationship. Int J Gynecol Cancer. 2015;25(7):1232–1238.
- Takeshita S, Todo Y, Furuta Y, et al. Prognostic factors for patients with brain metastasis from gynecological cancer: a significance of treatment-free interval of more than 6 months. Jpn J Clin Oncol. 2017;47(7):604–610.
- Pakneshan S, Safarpour D, Tavassoli F, et al. Brain metastasis from ovarian cancer: a systematic review. J Neurooncol. 2014;119(1):1–6.
- Piura E, Piura B. Brain metastases from ovarian carcinoma. ISRN Oncol. 2011;2011:527453.
- Morgan AJ, Giannoudis A, Palmieri C. The genomic landscape of breast cancer brain metastases: a systematic review. Lancet Oncol. 2021;22(1):e7–e17.
- Keskin S, Kucucuk S, Ak N, et al. Survival impact of optimal surgical cytoreduction in recurrent epithelial ovarian cancer with brain metastasis. Oncol Res Treat. 2019;42(3):101–106.
- Nasu K, Satoh T, Nishio S, et al. Clinicopathologic features of brain metastases from gynecologic malignancies: a retrospective study of 139 cases (KCOG-G1001s trial). Gynecol Oncol. 2013;128(2):198–203.
- Bahat Z, Cakmak VA, Cakir E. Brain metastasis from ovarian carcinoma: analysis of eight cases from a single radiotherapy center. Taiwan J Obstet Gynecol. 2020;59(5):711–717.
- Noordijk EM, Vecht CJ, Haaxma-Reiche H, et al. The choice of treatment of single brain metastasis should be based on extracranial tumor activity and age. Int J Radiat Oncol Biol Phys. 1994;29(4):711–717.
- Mintz AH, Kestle J, Rathbone MP, et al. A randomized trial to assess the efficacy of surgery in addition to radiotherapy in patients with a single cerebral metastasis. Cancer. 1996;78(7):1470–1476.
- Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280(17):1485–1489.
- Patchell RA, Regine WF. The rationale for adjuvant whole brain radiation therapy with radiosurgery in the treatment of single brain metastases. Technol Cancer Res Treat. 2003;2(2):111–115.
- Ledermann JA, Drew Y, Kristeleit RS. Homologous recombination deficiency and ovarian cancer. Eur J Cancer. 2016;60:49–58.
- Radu MR, Prădatu A, Duică F, et al. Ovarian cancer: biomarkers and targeted therapy. Biomedicines. 2021;9(6):693.