Introduction
Adenoid cystic carcinoma (ACC) is a rare head-and-neck malignancy, with a yearly incidence of 3–4.5 cases per million, most commonly observed in middle age women and involving salivary glands. Despite an apparently local indolent course, ACC has an aggressive long-term behavior, presenting with local or distant recurrence even several years after optimal primary tumor treatment, and with a rather poor prognosis (15-years overall survival rate <25%) [Citation1–3]. Distant metastases are observed in more than 50% of cases, even up to 10–20 years after the first diagnosis, being lung the most common site of metastatic involvement. 11C-methionine (11C-MET) positron emission tomography/computed tomography (PET/CT) is considered an effective imaging tool for assessing recurrent ACC, with tracer uptake reflecting increased amino acid metabolism associated with tumor growth [Citation4,Citation5]. However, 11C-MET PET/CT detection of histologically confirmed ACC pulmonary metastases has never been reported before.
Case description
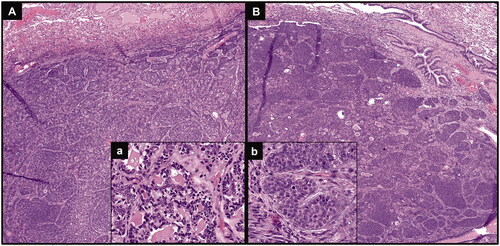
A 66-year-old woman was diagnosed with ACC of the right parotid gland and treated with total parotidectomy in October 2014 (pathological stage: pT2N0, according to TNM staging system, 7th edition); histology proved positive surgical margins and perineural invasion. Post-surgical 11C-MET PET/CT performed in December 2014 (whole-body acquisition from the vertex to midthighs, starting 10 min after intravenous injection of 345 MBq of 11C-MET) showed no evidence of disease spread. Adjuvant radio-chemotherapy treatment was then performed (from January to March 2015): conventionally fractionated radiotherapy was delivered by a linear accelerator using Volumetric Modulated Arc Therapy (VMAT) technique, for a total dose of 64.8 Gy (fractionation 1.8 Gy/day) to the post-operative parotid region and for a total dose of 50.4 Gy (fractionation 1.8 Gy/day) to the right laterocervical drainage lymph nodes, in association with 3 cycles of chemotherapy (Cisplatin 100 mg/mq q21). During oncological follow-up, in July 2017 a sub-centimetric pulmonary nodule (maximum axial diameter: 6 mm) was detected at CT in the left upper lobe; radiological monitoring was advised. It remained stable over time until September 2020, when a subsequent CT showed increased nodule size (maximum axial diameter: 26 mm) and appearance of a second lung nodule in the left lower lobe (maximum axial diameter: 16 mm). A 18F-fluorodeoxyglucose (18F-FDG) PET/CT was performed in October 2020 to metabolically characterize the lung findings, considered suspicious for malignancy due to the radiological progression: increased 18F-FDG uptake (SUVmax: 4.9) was noticed in the left upper lobe nodule, whereas only weak tracer uptake, lower than the mediastinal blood-pool activity, was evident in the other nodule; no other sites of abnormal 18F-FDG uptake were found (). Considering both the different 18F-FDG activity degree between the two nodules (the weaker active one not attributable to glucose-avid malignancy) and the previous ACC history, a subsequent 11C-MET PET/CT was performed (whole-body acquisition from the vertex to midthighs, starting 10 min after intravenous injection of 392 MBq of 11C-MET). With respect to 18F-FDG, an increased and similar 11C-MET activity (SUVmax: 4.1) was noticed in the left upper lobe nodule; conversely, an increased 11C-MET uptake (SUVmax: 4.9), significantly higher than 18F-FDG activity, was also evident in the lower lobe nodule, and no other abnormal findings were found (). Based on this combined functional pattern, both nodules were considered highly suspicious for ACC metastases and surgical resection (left upper lobectomy plus atypical resection of left lower lobe) was performed in November 2020. Histological examination confirmed metastases from the previous ACC in both the upper and the lower lobe nodule (). At last clinical follow-up (December 2021), the patient was alive with no evidence of disease.
Figure 1. Transaxial co-registered CT and fused images of 18F-FDG PET/CT showing increased tracer uptake in the left upper lung lobe nodule (A and B, arrows), but only weak glucose activity in the lower lobe nodule (C and D, arrows); maximum intensity projection image (E) showing no other sites of abnormal 18F-FDG uptake. At subsequent 11C-methionine PET/CT, transaxial co-registered CT and fused images showed increased and similar uptake of amino acidic tracer both in the left upper lung lobe nodule (F and G, arrows) and in the lower lobe nodule (H and I, arrows); maximum intensity projection image (L) showing no other sites of abnormal 11C-methionine uptake, so suggesting ACC oligo-metastatic lung involvement.
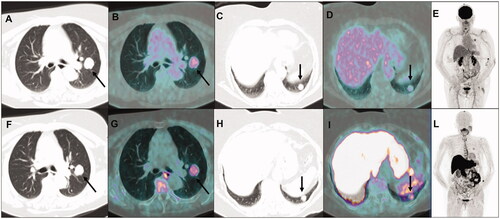
Figure 2. Hematoxylin & eosin staining of both lung nodules confirming metastases from the previous ACC, with basaloid neoplastic cells arranged in a predominantly solid architecture (A and B, original magnification 40×), presenting some cribriform areas, with glandular structures containing amorphous material (inset a; original magnification 200×) and some mitotic features (inset b; original magnification 400×).
Discussion
In the oncological setting, especially when compared to the widely used 18F-FDG, the general availability of 11C-MET as radiotracer for PET cancer imaging is limited by the short half-life of 11C (20 min vs. 110 min of 18F): indeed, this represents a significant drawback, implying the need of on-site cyclotron for tracer production and making 11C-MET not commercially available. However, in PET/CT centers provided of a local cyclotron, PET imaging with 11C-MET is used in patients with various oncological diseases, mainly represented by adenoid cystic carcinoma, central nervous system malignancies (primary brain tumors and brain metastases) and, more recently, by plasma cell malignancies. In the context of a rare tumor as ACC, our case firstly describes 11C-MET PET/CT detection of histologically confirmed pulmonary metastases, presenting several years after primary tumor treatment. Moreover, the 11C-MET functional pattern, suggestive for ACC oligo-metastatic lung involvement with no extra-pulmonary sites of disease, allowed to address the patient to surgical resection with curative intent, so highlighting the role of 11C-MET imaging in the diagnostic and therapeutic work-up algorithm of ACC patients.
Appearance of distant metastases in the disease course of ACC patients is very common, with lung representing the most common site of metastatic involvement (75% of cases). To early recognize pulmonary metastases, ideally at an oligo-metastatic status and before widespread organs involvement, takes peculiar relevance in a therapeutic and prognostic perspective, especially considering the potential long life expectancy of ACC patients (due to slow tumor growth and generally young patients’ age). Indeed, although there is no general consensus on the management of metastatic disease, a complete disease control might be achieved by lung metastasectomy or loco-regional treatment (including surgery and radiotherapy) [Citation3]. Moreover, lung metastases are associated with a more favorable prognosis than other metastatic sites such as bone and liver (median overall survival: 32-46 months vs 8-20 months) [Citation6,Citation7]. In our case, stated that 11C-MET PET/CT is an effective modality for assessing ACC recurrence (relying on increased amino acid tumor metabolism)[Citation4,Citation5], the increased 11C-MET uptake in both lung nodules was considered a clue of their metastatic nature. Conversely, a discordant degree of 18F-FDG activity was observed between the lung nodules and when compared to 11C-MET (one showing only weak glucose metabolism). This finding reflects the generally known low sensitivity of 18F-FDG in ACC, consistent with the common low glucose-avidity and slow tumor growth, with only some reports demonstrating a diagnostic value of 18F-FDG PET/CT [Citation8,Citation9]. In addition, 11C-MET PET/CT allowed to exclude extra-pulmonary sites of disease, so addressing the patient to curative surgical resection, with impact on both treatment planning and prognostic stratification. Finally, the slow growth rate of lung nodules in our patient was concordant with the reported long doubling time of pulmonary metastases from ACC (range: 86–1064 days, average: 393 days), much longer than those from other malignancies, and interestingly suggesting that metastases at the cellular level could occur even prior to primary ACC clinical presentation [Citation10].
Ethical approval
The patient gave her written informed consent for reporting and publishing her case.
Acknowledgment
The authors have no funding to declare.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Due to the nature of this research (case report), the data are not publicly available since containing personal information that could compromise the privacy of the patient.
References
- Garg M, Tudor-Green B, Bisase B. Current thinking in the management of adenoid cystic carcinoma of the head and neck. Br J Oral Maxillofac Surg. 2019;57(8):716–721.
- Coca-Pelaz A, Rodrigo JP, Bradley PJ, et al. Adenoid cystic carcinoma of the head and neck-an update. Oral Oncol. 2015;51(7):652–661.
- Girelli L, Locati L, Galeone C, et al. Lung metastasectomy in adenoid cystic cancer: is it worth it? Oral Oncol. 2017;65:114–118.
- Lindholm P, Leskinen-Kallio S, Grénman R, et al. Evaluation of response to radiotherapy in head and neck cancer by positron emission tomography and [11C]methionine. Int J Radiat Oncol Biol Phys. 1995;32(3):787–794.
- Toubaru S, Yoshikawa K, Ohashi S, et al. Accuracy of methionine-PET in predicting the efficacy of heavy-particle therapy on primary adenoid cystic carcinomas of the head and neck. Radiat Oncol. 2013;8:143.
- Cavalieri S, Mariani L, Vander Poorten V, et al. Prognostic nomogram in patients with metastatic adenoid cystic carcinoma of the salivary glands. Eur J Cancer. 2020;136:35–42.
- van Weert S, Bloemena E, van der Waal I, et al. Adenoid cystic carcinoma of the head and neck: a single-center analysis of 105 consecutive cases over a 30-year period. Oral Oncol. 2013;49(8):824–829.
- Parihar AS, Vadi SK, Mittal BR, et al. Adenoid cystic carcinoma of buccal mucosa: Role of 18F-Fluorodeoxyglucose positron emission tomography/computed tomography in the detection and biopsy of pulmonary metastases and assessment of treatment response. Indian J Nucl Med. 2019;34(1):71–73.
- Wong WL. PET-CT for staging and detection of recurrence of head and neck cancer. Semin Nucl Med. 2021;51(1):13–25.
- Umeda M, Nishimatsu N, Masago H, et al. Tumor-doubling time and onset of pulmonary metastasis from adenoid cystic carcinoma of the salivary gland. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88(4):473–478.
