Introduction
Primary diffuse large B-cell lymphoma (DLBCL) of the central nervous system (PCNSL) is a distinct entity of DLBCL that arises in the brain, spinal cord, leptomeninges and eyes [Citation1]. The disease affects predominantly elderly patients with increasing incidence during the last three decades [Citation2] and with a dismal outcome compared to systemic DLBCL (sDLBCL) [Citation3]. The established prognostic impact of cell of origin (COO) and protein expression and/or genetic alterations of MYC, BCL2 and BCL6 in sDLBCL is not applicable in PCNSL. Several studies found the majority of PCNSL cases to belong to the non-GCB subtype, but with no prognostic impact on survival [Citation4,Citation5]. On the other hand, conflicting results were reported regarding the prognostic impact of genetic alterations and protein expression of MYC, BCL2 and BCL6 in PCNSL [Citation6].
Cancer immunotherapy has successfully launched a new era in oncology by using immune checkpoint inhibitors and immunological tolerance modifiers in treating various malignancies. However, the results in hematological malignancies have been disappointing, except for classical Hodgkin lymphoma (cHL), probably based on the important role of the PD-1/PDL-1 axis in cHL [Citation7]. Trials of combined PD-1 and IDO1 inhibitors have been evaluated in order to restore T-cell function and aid killing of cancer cells [Citation8–10]. The role of PD-1/PD-L1 expression in PCNSL has gained increasing attention since an initial report of successful therapy with a PD-1 inhibitor [Citation11]. The protein expression of PD-1 and its ligands, as well as IDO1 have been explored in different types of solid and hematological malignancies. However, only a few published studies evaluated the expression of PD-L1 and PD-L2 in PCNSL with contradictory results regarding the association of these proteins with survival [Citation12,Citation13]. No previous studies were published concerning the prognostic impact of IDO1 protein expression in PCNSL.
In a previous study from our group, we found a significant association between gene and protein expression of IDO1 and protein expression of PD-L1 in the tumor microenvironment of PCNSL [Citation14]. As a follow-up to our previous study, we expanded our cohort and aimed to evaluate the protein expression of PD-1, PD-L1 and PD-L2 in relation to the clinical characterization, immunophenotypic and molecular features of formalin-fixed, paraffin-embedded (FFPE) tumor biopsies of PCNSL.
Material and methods
Patients
We evaluated 174 PCNSL patients (whole cohort), including our previous cohort [Citation14] and 83 PCNSL patients diagnosed at the Department of Pathology, Karolinska University Hospital, between 1992–2019. FFPE diagnostic biopsies were reviewed by two hematopathologists (RMA, LS), and initial diagnosis of PCNSL was confirmed according to the 2017 WHO classification of Tumors of Hematopoietic and Lymphoid Tissues [Citation1,Citation15]. We performed analyses on tissue microarrays (TMA) containing two cores of one mm in diameter per case in 49 PCNSL patients with sufficient tumor tissue in whom curative treatment was intended and clinical data was available (TMA cohort). This study was approved by the Swedish Ethical Review Authority (dnr 2019-00087, 233-2014, 246-2008).
Methods
Immunohistochemistry and FISH for MYC, BCL2 and BCL6 were performed and evaluated as previously described [Citation14].
Treatment
Curative treatment with chemotherapy with and without high dose of methotrexate (MTX) and/or radiotherapy was intended in 110 patients.
Statistical analyses
Cut off values for low and high proportions of PD-1, PD-L1 and PD-L2 positive cells were determined with receiver operating characteristic curves with Youden’s index calculated. Optimal cut off values were ≥2.5% PD-1, ≥85% PD-L1 and ≥8% PD-L2 for leukocytes, and ≥1% PD-1, >80% PD-L1 and >60% PD-L2 for tumor cells. Overall survival (OS) was defined as time from diagnosis to time at last follow up or death from any cause. All patients either died due to lymphoma or with lymphoma. Patients who were alive at last follow-up were censored. Survival curves and univariable analyses were analyzed with the Kaplan–Meier method, the log-rank test and Cox proportional hazards regression in the TMA cohort. Statistically significant variables in the univariable survival analyses were included in the multivariable Cox regression analysis. Chi-square or Fisher’s exact test were used to compare tabulated values and mean differences were compared with the Wilcoxon rank-sum test between the whole cohort and the TMA cohort. P-values <0.05 were considered significant. Statistical analyses were performed with R Studio 1.1.383 (www.r-project.org).
Results
Clinicopathological characteristics
In the whole cohort, median age was 66 and 46% were female. At diagnosis, 45% had a MSKCC score >2 and 50% had elevated lactate dehydrogenase. According to the Hans algorithm, 86% were of non-germinal center B-cell origin (non-GCB). High protein expression of MYC, BCL2 and BCL6 was detected in 48%, 90% and 59%, respectively. Double protein expression of MYC and BCL2 was detected in 48% of cases. There was no case with translocation of MYC or BCL2, while BCL6 translocation was found in 19% ().
Table 1. Clinicopathological features of patients with PCNSL in the whole cohort, TMA cohort and according to high and low expression of PD-L1 on leukocytes in the TMA cohort.
There were no differences between the whole cohort and the TMA cohort regarding clinicopathological variables. Clinical data on type of treatment was available in 45 patients in the TMA cohort, where 35 were treated with regimens including methotrexate. High protein expression of PD-1, PD-L1 and PD-L2 on leukocytes was detected in 47%, 57% and 51%, respectively. Expression of IDO1 on leukocytes was detected in 28%. Patients with a high proportion of PD-L1+ leukocytes were younger, and more frequently had a high proportion of PD-L1+ tumor cells, high proportion of PD-L2+ leukocytes and IDO1+ leukocytes compared to patients with a low proportion of PD-L1+ leukocytes ().
Survival analyses
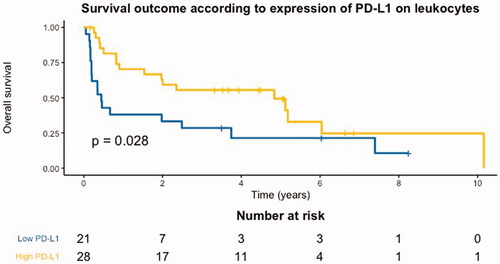
Five-year OS was 25% in the whole cohort and 41% in the TMA cohort. In the TMA cohort, 34 patients died during follow-up and patients with high proportions of PD-L1+ leukocytes had a superior 5-year OS of 54%, compared to patients with low proportions of PD-L1+ leukocytes with a 5-year OS of 24% (). Age (continuous variable) (hazard ratio (HR)=1.03, [95% confidence interval (CI) 0.18–0.79], p = 0.01), treatment with methotrexate (HR = 0.23, [95% CI 0.13–0.41], p < 0.001), and high proportions of PD-L1+ leukocytes (HR = 0.47, [95% CI 0.24–0.94], p = 0.03) and PD-L2+ leukocytes (HR = 0.37, [95% CI 0.18–0.79], p = 0.01) were associated with OS in univariable survival analyses, while all other clinicopathological variables were not statistically significantly associated with OS. In a multivariable analysis, only a high proportion of PD-L1+ leukocytes (HR = 0.35, [95% CI 0.13–0.91], p = 0.03) and treatment with methotrexate (HR = 0.17, [95% CI 0.06–0.46], p < 0.001) remained statistically significant.
Discussion
In this study, 49 PCNSL patients were treated with curative intent and had tumor material available for pathological analyses. We assessed the protein expression of PD-1 ligands on leukocytes and tumor cells and found that high proportions of PD-L1+ and PD-L2+ leukocytes were associated with superior outcome, while only PD-L1+ leukocytes were associated with superior outcome in a multivariable analysis. High proportions of PD-L1+ and PD-L2+ tumor cells had no prognostic impact. This may be explained by the different mechanisms, which underlie protein expression of PD-L1 and PD-L2 on tumor cells versus immune cells, though both cell types can regulate PD-1 ligands and contribute to immune suppression Citation16,Citation17.
The role of PD-1 and its ligands in PCNSL has been evaluated by a limited number of studies. Lower rate of high proportions of PD-L1+ and PD-L2+ tumor cells than leukocytes was reported by others [Citation12,Citation13]. An adverse prognostic impact of PD-1 expression in PCNSL but no significant association of PD-L1 and PD-L2 expression with survival [Citation18] has been reported. In addition, a superior outcome among PCNSL patients with PD-L1+ tumor cells and no significant association of PD-L1+ leukocyte with survival was found [Citation13]. Similar to our finding, Furuse et al revealed that patients with high expression of PD-L1 on macrophages had superior outcome compared to those with low PD-L1 expression on macrophages [Citation12]. However, these studies used different antibody clones and different methods to estimate the expression of PD-1 and its ligands, and calculate cut off values, which may explain the discrepant results. Furuse et al however used the same antibody clone and assessed the protein expression of PD-1 ligands in a similar way to our study.
A historical improvement of survival in PCNSL was achieved since the introduction of MTX in the treatment regimens, though significant neurotoxic side effects were reported upon prolonged survival [Citation19,Citation20]. In our cohort, both treatment with MTX and high proportion of PD-L1+ leukocytes were associated with superior outcome in a multivariable analysis. The role of immunosuppressive mechanism of action of MTX with the immunosuppressive role of PD-L1 in the tumor microenvironment in PCSNL remains to be resolved.
IDO1 was investigated in PCNSL in only a few studies [Citation21]. In our previous study, we identified a significant association between gene and protein expression of IDO1 and protein expression of PD-L1 on leukocytes [Citation14]. In the current study, there was still a significant association between IDO1 and PD-L1 protein expression on leukocytes; however, protein expression of IDO1 on leukocytes still had no prognostic impact.
The majority of our cases belong to the non-GCB subtype according to the Hans algorithm, and there was no significant difference in survival between the GCB- and the non-GCB subtypes, which is consistent with previous studies [Citation4,Citation5,Citation22]. Protein expression of MYC, BCL2, and BCL6 was frequent in our cohort, while the rate of rearrangement of their corresponding genes was very low, except for the BCL6 gene, which was rearranged in contrast to MYC and BCL2 [Citation23]. The lack of prognostic impact of single and/or double protein expression of MYC, BCL2, BCL6 or translocation of BCL6 in our study differs from previous studies. Villa et al reported inferior survival among PCNSL patients with BCL6 rearrangement while protein expression of MYC and/or BCL2 was not associated with survival [Citation4]. Chen et al identified protein expression of BCL2 as an adverse prognostic factor in their PCNSL cohort [Citation24]. Hatzl et al described that double protein expression of MYC and BCL2 was associated with inferior outcome in their PCNSL cohort [Citation25]. However, a meta-analysis of 31 different studies revealed contradictory results regarding the prognostic impact of MYC, BCL2 and BCL6 expression in PCNSL [Citation6]. Like our study, most of the previous studies were based on small cohorts. In addition, most studies had different clinical treatments, and used different cutoff values to assess protein expression of MYC, BCL2, and BCL6.
An important limitation in our cohort was that a substantial proportion of the patients were excluded from the immunophenotypic and molecular analyses due to insufficient biopsy samples. In addition, we excluded patients who did not receive curative treatment despite available TMA when assessing survival. In the TMA cohort, 78% of patients treated with curative intent received MTX, which is due to the long time period patients were diagnosed (1992–2019), where patients treated in the earlier time period received non-contemporary treatment regimens. In addition, our Cox regression analyses should be interpreted cautiously, due to the low number of cases with tumor material and treated with curative intent (n = 49), which limits the statistical power of our study. Also, in the multivariable Cox regression analysis four variables were included while only 34 events occurred in the TMA cohort, which adds to the potential risk of type II error in the survival analyses. However, we consider that our cohort was not significantly affected by selective bias, since the clinical and immunophenotypic features were similar in the TMA cohort compared to the whole cohort and despite the numerically better survival in the TMA cohort compared to the whole cohort which can be explained by the higher frequency of patients treated with MTX within the TMA cohort () [Citation14].
In conclusion, we identified a significant superior outcome in a small cohort of PCNSL patients with high proportion of PD-L1+ leukocytes. In addition, we explored a significant association between the expression of PD-1 ligand, PD-L1 and IDO1 on leukocytes. These findings may indicate the need of further investigations of larger cohorts to study the role of the immune system in response to treatment of PCNSL and to identify new prognostic markers in the era of new trails of treatment with immunotherapies as PD-1 blockade and IDO1 inhibitors.
Supplemental Material
Download MS Word (27.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Swerdlow SH. WHO classification of tumours of haematopoietic and lymphoid tissues. : International Agency for Research on Cancer (IARC) 2016.
- Eloranta S, Branvall E, Celsing F, et al. Increasing incidence of primary Central nervous system lymphoma but no improvement in survival in Sweden 2000-2013. Eur J Haematol. 2018;100(1):61–68.
- Langner-Lemercier S, Houillier C, Soussain C, et al. Primary CNS lymphoma at first relapse/progression: characteristics, management, and outcome of 256 patients from the French LOC network. NEUONC. 2016;18(9):1297–1303.
- Villa D, Tan KL, Steidl C, et al. Molecular features of a large cohort of primary central nervous system lymphoma using tissue microarray. Blood Adv. 2019;3(23):3953–3961.
- Camilleri-Broet S, Criniere E, Broet P, et al. A uniform activated B-cell-like immunophenotype might explain the poor prognosis of primary central nervous system lymphomas: analysis of 83 cases. Blood. 2006;107(1):190–196.
- Ge L, Lu S, Xu L, et al. MYC, BCL2, and BCL6 expression as prognostic indicators in primary Central nervous system lymphoma: a systematic review and meta-analysis. Clin Neurol Neurosurg. 2021;208:106838.
- Xie W, Medeiros LJ, Li S, et al. PD-1/PD-L1 pathway and its blockade in patients with classic Hodgkin lymphoma and non-Hodgkin large-cell lymphomas. Curr Hematol Malig Rep. 2020;15(4):372–381.
- Hatic H, Sampat D, Goyal G. Immune checkpoint inhibitors in lymphoma: challenges and opportunities. Ann Transl Med. 2021;9(12):1037.
- Wu RY, Kong PF, Xia LP, et al. Regorafenib promotes antitumor immunity via inhibiting PD-L1 and IDO1 expression in melanoma. Clin Cancer Res. 2019;25(14):4530–4541.
- Epacadostat shows value in two SCCHN trials. Cancer Discovery. 2017;7(9):OF2.
- Nayak L, Iwamoto FM, LaCasce A, et al. PD-1 blockade with nivolumab in relapsed/refractory primary Central nervous system and testicular lymphoma. Blood. 2017;129(23):3071–3073.
- Furuse M, Kuwabara H, Ikeda N, et al. PD-L1 and PD-L2 expression in the tumor microenvironment including peritumoral tissue in primary Central nervous system lymphoma. BMC Cancer. 2020;20(1):277.
- Hayano A, Komohara Y, Takashima Y, et al. Programmed cell death ligand 1 expression in primary Central nervous system lymphomas: a clinicopathological study. Anticancer Res. 2017;37(10):5655–5666.
- Abdulla M, Alexsson A, Sundstrom C, et al. PD-L1 and IDO1 are potential targets for treatment in patients with primary diffuse large B-cell lymphoma of the CNS. Acta Oncol. 2021;60(4):531–538.
- Campo E, Swerdlow SH, Harris NL, et al. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117(19):5019–5032.
- Chen S, Crabill GA, Pritchard TS, et al. Mechanisms regulating PD-L1 expression on tumor and immune cells. J Immunother Cancer. 2019;7(1):305.
- Yearley JH, Gibson C, Yu N, et al. PD-L2 expression in human tumors: Relevance to anti-PD-1 therapy in cancer. Clin Cancer Res. 2017;23(12):3158–3167.
- Cho H, Kim SH, Kim SJ, et al. Programmed cell death 1 expression is associated with inferior survival in patients with primary Central nervous system lymphoma. Oncotarget. 2017;8(50):87317–87328.
- Grommes C, DeAngelis LM. Primary CNS lymphoma. J Clin Oncol. 2017;35(21):2410–2418.
- Grommes C, Rubenstein JL, DeAngelis LM, et al. Comprehensive approach to diagnosis and treatment of newly diagnosed primary CNS lymphoma. Neuro Oncol. 2019;21(3):296–305.
- Miyasato Y, Takashima Y, Takeya H, et al. The expression of PD-1 ligands and IDO1 by macrophage/microglia in primary Central nervous system lymphoma. J Clin Exp Hematop. 2018;58(2):95–101.
- Hattab EM, Martin SE, Al-Khatib SM, et al. Most primary central nervous system diffuse large B-cell lymphomas occurring in immunocompetent individuals belong to the nongerminal center subtype: a retrospective analysis of 31 cases. Mod Pathol. 2010;23(2):235–243.
- Montesinos-Rongen M, Zuhlke-Jenisch R, Gesk S, et al. Interphase cytogenetic analysis of lymphoma-associated chromosomal breakpoints in primary diffuse large B-cell lymphomas of the Central nervous system. J Neuropathol Exp Neurol. 2002;61(10):926–933.
- Chen Y, Chen H, Chen L, et al. Immunohistochemical overexpression of BCL-2 protein predicts an inferior survival in patients with primary Central nervous system diffuse large B-cell lymphoma. Medicine (Baltimore). 2019;98(45):e17827.
- Hatzl S, Posch F, Deutsch A, et al. Immunohistochemistry for c-myc and bcl-2 overexpression improves risk stratification in primary Central nervous system lymphoma. Hematol Oncol. 2020;38(3):277–283.