Abstract
Background
Second primary cancer (SPC), defined as a metachronous solid cancer resulting from neither a recurrence of the primary cancer nor a metastasis, is a leading long-term cause of death for survivors of primary oral squamous cell carcinoma (OSCC). This study examined the risk of SPC following treatment of primary OSCC.
Materials and Methods
This semi-national, population-based, retrospective study included all patients with primary OSCC treated with curative intent in Eastern Denmark in 2000–2014. The presence of SPC was confirmed from medical records and the Danish Pathology Data Bank. The rate of SPC was compared to the occurrence of any cancer in the Eastern Danish population using data from the Danish Cancer Registry.
Results
A total of 936 patients with primary OSSC were enrolled. Of these, 219 patients (23%) were diagnosed with SPC during the follow-up (median 8.9 years, IQR: 5.4–12.6 years). The rate of SPC was four times higher than the occurrence of any cancer among the Eastern Danish population i.e., with a standardized incidence ratio (SIR) of 4.13 (95%CI: 3.55–4.80). SPCs were most frequently found in head and neck region (n = 97, SIR = 43.6), lower respiratory organs (n = 38, SIR = 5.6) and gastrointestinal organs (n = 33, SIR = 3.2) with increased SPC rates in all locations. Among patients who developed SPC within the study period the median time from OSCC to the first SPC was 4.4 years (IQR: 2.5–6.2). Significant associations were found between both smoking and excessive alcohol consumption after treatment of OSCC and the risk of SPC.
Conclusions
A noteworthy increased rate of SPC following treatment of primary OSCC was found, especially in the head and neck region and in the lungs. Healthcare professionals should be aware of this increased risk.
Background
Worldwide, oral cavity cancer is the most common head and neck cancer (HNC) accounting for 40% of all new HNCs and 2% of all diagnosed malignancies with an incidence of more than 350,000 new cases a year [Citation1]. Oral squamous cell carcinomas (OSCC) constitute the vast majority of cancers in the oral cavity. In Denmark, oral cavity cancer (excluding lip cancer) is the second most common head and neck cancer i.e., only oropharyngeal cancer is more common due to the impact of HPV [Citation2]. Both the incidence and survival rates of OSCC have increased in the past decades in Denmark [Citation2,Citation3] comparable with other countries [Citation4,Citation5]. Due to improvements in survival and the aging population, the occurrence of second primary cancer (SPC) has risen considerably [Citation6]. SPC is a leading long term cause of death for survivors of primary OSCC [Citation7,Citation8], and data from Europe and Northern America indicates that the risk of developing SPC among HNC patients is twice the risk of developing any cancer among the general population [Citation9–11]. Several potential risk factors in developing SPC following primary OSCC are identified and include smoking [Citation12–14], alcohol [Citation12,Citation13], genetic susceptibility [Citation15] and possibly previous radiotherapy [Citation16].
All Danish citizens have free access to diagnostics and treatments at public university cancer centers, financed by general tax revenue. As registration in the Danish Pathology Data Bank is mandatory, we have an ideal basis for retrospective and large cohort analyses. In Denmark, it is recommended for patients treated for OSCC to have follow-up visits for at least a five-year period according to the guidelines of The Danish Head and Neck cancer Study Group (DAHANCA).
The purpose of this study was to evaluate the risk of SPC following a primary OSCC treated with curative intent and assess baseline characteristics for patients with and without SPC.
Material and methods
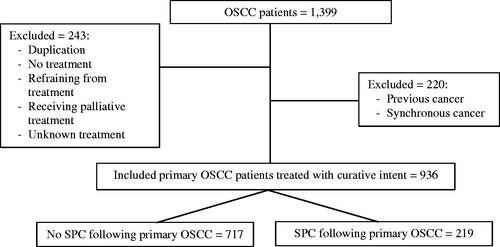
This retrospective study included all patients diagnosed with primary OSCC receiving treatment with a curative intent in the Eastern Region of Denmark, primarily at Rigshospitalet, in the period 2000-2014. Information on these patients was obtained from the Copenhagen Oral Cavity Squamous Cell Carcinoma (COrCa) database [Citation17]. Registered OSCC patients with any previous or synchronous cancer (i.e., simultaneously or within six months of the primary tumor) were excluded ().
Figure 1. Flowchart of included patients from the Copenhagen Oral Cavity Squamous Cell Carcinoma database (COrCa) with reasons for exclusion.
SPC in any location was defined as a metachronous solid cancer (i.e., more than six months after diagnosis of OSCC) and not as a result of neither a recurrence of the primary OSCC nor a metastasis [Citation18]:
Metachronous secondary cancer developing in a different location than the oral cavity (and not being a metastasis) or being of non-squamous cell origin was diagnosed as a SPC.
Locally recurrent OSCC was excluded using the Odense-Birmingham definition [Citation19]. If the metachronous secondary cancer was a squamous cell carcinoma and located in the oral cavity it was diagnosed as a SPC if occurring more than three centimeters from the primary OSCC separated by healthy tissue (and not being a metastasis) or occurring more than three years after the primary OSCC at the same tumor site [Citation19].
Data from the COrCA database comprised baseline information of the patients at time of diagnosis. The data included the following information: sex, age at diagnosis, subsite of OSCC (floor of mouth, oral tongue, gingiva and ‘other subsite’), TNM classification, Union for International Cancer Control (UICC) stage (7th edition), treatment, tobacco smoking (before and after treatment of OSCC), alcohol consumption (before and after treatment of OSCC), and Charlson Comorbidity Index (CCI). The CCI index is a comorbidity summary measure, providing a single number description of the overall comorbidity status for the patient, excluding the primary OSCC diagnosis. The CCI was calculated for each patient based on data from the Danish National Patient Register, comprising diagnoses registered during hospital visits [Citation20].
Patients with SPC were further examined providing data on the SPC location and subdivided into head and neck, lower respiratory area and intrathoracic region, gastrointestinal organs, reproductive organs, skin (excluding basal-cell carcinoma) and “other locations” (incl. cancer in breast, urinary tract, bones and lymphomas). Second primary cancer in the head and neck region was further subdivided into oral cavity, pharynx, larynx and sinuses. Further, data included the time from OSCC to SPC and the time from SPC to either death or end of follow-up monitoring. Data on SPC obtained from the database was validated by reviewing the medical records and the Danish Pathology Data Bank.
Statistical analysis
Statistical analyses were performed using R software (version 3.6.1). Patients with SPC following a diagnosis of primary OSCC were compared to patients without SPC to examine the differences in baseline characteristics of the two groups.
Categorical variables were reported as frequencies and continuous variables as median values with an interquartile range (IQR). Potential associations were expressed as Hazard Ratios (HR) using univariate and multivariate cox regression analysis. The multivariate cox regression analysis included the variables of sex, age at diagnosis, subsite of OSCC (i.e., floor of mouth, oral tongue, gingiva, and others), tobacco smoking (before and after treatment of OSCC), alcohol consumption (before and after treatment of OSCC), as well as the CCI and UICC stage.
A Standardized Incidence Ratio (SIR) with 95% confidence intervals [95% CI] was calculated overall and for different SPC locations (head and neck, lower respiratory and intrathoracic region, gastrointestinal organs, reproductive organs, skin and “other locations”) with data from the Danish Cancer Registry (Danish Health Data Authority) [Citation21]. The risk among patients treated for primary OSCC were calculated six months from diagnosis (SPC is impossible within the first six months due to the exclusion of synchronous cancer) until either date of death, end of follow-up monitoring or diagnosis of a SPC. The expected number of cancers in the Eastern Denmark population during 2000–2014 was used as a reference and estimated with data from the Danish Cancer Registry [Citation21]. The number of cases for the population of Eastern Denmark was estimated by filtering number of malignancies per 100.000 inhabitants for different types of cancer in different calendar years for the population of Eastern Denmark in the Danish Cancer Registry. Standardized Incidence Ratio (SIR) was obtained by dividing the observed number of cases of SPC by the number expected in the population of Eastern Denmark. This provided an estimate of the rate of OSCC patients developing a SPC relative to the occurrence of cancer in the Eastern Danish population. The probability of overall survival (1-, 3- and 5-year survival proportion) following the patients’ first diagnosis of SPC was estimated by using a Kaplan-Meier analysis.
Results
Baseline characteristics
A total of 936 patients diagnosed with primary OSCC and treated with curative intent were included (), with a median age of 61 years (IQR 54–69 years, range 25–95 years). The majority was men (64%, n = 596) and the median follow-up time was 8.9 years (IQR 5.4–12.6 years, range 1.89–18.3 years). Most patients were treated with surgery as single-modality treatment, (n = 443, 47%), or surgery with adjuvant radiotherapy (n = 338, 36%) ().
Table 1. Baseline characteristics of patients with and without second primary cancer following primary OSCC diagnosed at Rigshospitalet, Copenhagen, Denmark during the period 2000–2014.
SPC following OSCC
Of the 936 included patients a total of 219 patients (23%) were diagnosed with SPC (). The majority of the 219 patients was diagnosed with a single SPC in the study period (83%, n = 182), as the rest was diagnosed with two or more SPCs (17%, n = 37). The median follow-up time after diagnosis of OSCC for patients with SPC was 10.2 years (IQR 6.8–13.2, range 2.2–18.3). Among patients who developed SPC within the study period, the median time from OSCC to the first SPC was 4.4 years (IQR 2.5–6.2, range 0.5–14.4).
Table 2. Characteristics of SPC following primary OSCC diagnosed at Rigshospitalet, Copenhagen, Denmark during the period 2000–2014.
The most frequent location of the first SPC for the 219 patients were the head and neck region (44%, n = 97), especially secondary oral cancer (32%, n = 69) and pharyngeal cancer (11%, n = 23) (). The majority of the secondary oral cancer occurred more than three years after primary OSCC (n = 63) and most frequently in a different oral subsite than the primary OSCC (n = 44).
The univariate analysis showed a significant association between the risk of SPC after treatment of OSCC and smoking and excessive alcohol consumption, respectively (). This association was not found in the multivariate analysis. Both the univariate and the multivariate analysis showed a significant association between advanced stage cancer (UICC IV) and a lower risk of SPC. No association was found between the risk of SPC and CCI ().
The 1-year overall survival proportion after diagnosis of SPC following a primary OSCC was 60.2% (95%CI 53.9–67.2), while the 3- and 5-year overall survival proportion was respectively 41.8% (95%CI 35.3–49.5) and 32.8% (95%CI 26.3–41.0) (, Supplementary Figure 1).
The rate of SPC
The rate of SPC was four times higher than the occurrence of any cancer in the Eastern Danish population with an SIR of 4.13 (95%CI: 3.55–4.80) (). The rate of SPC was increased in multiple locations, especially in the head and neck region, but also in the lungs and gastrointestinal organs.
During the follow-up 97 patients developed SPC in the head and neck region which was an increased rate compared to the occurrence of head and neck cancer in the Eastern Danish population with a SIR of 43.61 (95%CI: 37.48–50.73). An increased rate of cancer was also found in the lower respiratory area (SIR 5.57, 95%CI: 4.79–6.48) and gastrointestinal organs (SIR 3.17, 95%CI: 2.72–3.69) ().
Discussion
This study found a four-fold increased rate of SPC following primary OSCC compared to the occurrence of any cancer among the Eastern Danish population. Previous studies have also showed increased rate of SPC following diagnosis of OSCC with a variation in different populations from SIR 1.47 in Korea [Citation22] to 1.85 in Finland [Citation23]. The higher rate in our study might be explained by the inclusion of only patients treated with curative intent as well as the longer follow-up monitoring. Similar to previous studies, SPCs following a primary OSCC were most commonly found in the head and neck region (the majority being a secondary OSCC) and in the lungs [Citation9–11]. We observed a noteworthy increased rate of head and neck cancer (SIR 43.6) in accordance with previous findings (SIR 26.2) [Citation24]. The higher rate of SPC in the head and neck region in our study might be explained partly by the inclusion of only patients treated with curative intent and mostly because of the difference in SPC definition. We defined SPC in the oral cavity as either occurring more than three centimeters from the primary OSCC or occurring more than three years after primary OSCC (and not being a metastasis) [Citation19], while others defined SPC in oral cavity without a distance criteria and only if occurring more than 5 years after primary OSCC [Citation24].
Previous studies have found an increased risk of SPC following primary OSCC for patients with high intakes of alcohol and tobacco, especially for current smokers who smoked more than 20 cigarettes per day [Citation13,Citation14] and for patients continuing to use tobacco and alcohol after treatment of a HNC [Citation12]. Our study found similar significant associations in the univariate analysis for patients continuing the use of tobacco and excessive alcohol consumption after treatment of primary OSCC. The multivariate analysis found no association between neither smoking status nor alcohol consumption (neither before nor after treatment of OSCC) and the risk of SPC. The lack of association in the multivariate analysis was unexpected since most SPCs appear in areas where smoking is a known risk factor of cancer, including lung cancer and various squamous cell carcinomas in the head and neck region [Citation25]. A possible explanation for the lack of association in the multivariate analysis might be insufficient information on the amount of daily tobacco consumption and the duration of tobacco smoking. Likewise, this lack of information applies to alcohol consumption, which only differentiated between excessive, normal, and no alcohol consumption. Further, as seen in , a noteworthy part of patients had an unknown alcohol (32%, n = 304) and smoking (26%, n = 243) status after treatment for OSCC.
Most patients were treated with surgery as single-modality treatment or with adjuvant radiotherapy. There was no statistical analysis on treatment and the risk of SPC, since many factors influence the choice of treatment. Previous findings indicate that radiation therapy of primary OSCC might be a risk factor in the development of solid SPC, but not until 10 years of follow-up [Citation16]. Therefore, this was not examined in this study.
An association was demonstrated between advanced stage cancer upon the diagnosis of OSCC and a lower risk of SPC. This might be explained by higher mortality related to advanced stage cancer [Citation4].
It is well known that SPC is a leading long term cause of death for survivors of primary OSCC [Citation7,Citation8] with previous findings showing a median survival of 12 months after diagnosis of SPC following a primary HNC [Citation26]. The overall survival proportion after diagnosis of SPC following primary OSCC in this study was more encouraging, with a 1-year survival proportion of 60%, a 3-year survival proportion of 42% and a 5-year survival proportion of 33%, which might be explained by the inclusion of only OSCC patients treated with curative intent.
A limitation to this study was the retrospective study design, which relied on the accuracy of the Danish Health Data Authority and the COrCa-database. There is a risk of misclassification of SPCs as metastases (underestimation) and vice versa (overestimation). The risk of overestimation was minimized by reviewing the medical records of patients with SPC, thereby validating the data on SPC obtained from the database. The follow-up period was relatively short (with a median of 8.9 years), although longer than most previous studies [Citation27]. A short follow-up period lead to a risk of underestimating SPC but might be of less importance since previous studies have shown that most SPCs appear within five years from diagnosis of OSCC [Citation23]. The noteworthy part of patients with unknown alcohol and smoking status after treatment of OSCC may have led to the lack of association between smoking and alcohol and the risk of SPC in the multivariate analysis.
The strengths of the study were the inclusion of all patients treated with curative intent in the Eastern Denmark region, comprising nearly half the approximately 5.8 million inhabitants. A further strength was the information on numerous parameters for all patients diagnosed and/or treated for OSCC in the COrCA-database.
With a median follow-up of nine years this study identified 219 cases (23%) with SPC among 936 patients with a primary OSCC treated with curative intent in Eastern Denmark in 2000–2014. OSCC patients showed a four-fold rate (SIR 4.13) of SPC compared to the occurrence of any cancer among the Eastern Danish population. Among patients who developed SPC within the study period, the median time from OSCC to the first SPC was 4.4 years. Healthcare professionals should be aware of the increased rate of SPC following primary OSCC, especially in the head and neck region and in the lungs.
Supplemental Material
Download MS Word (92.1 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
Raw data were generated at Department of Otorhinolaryngology, Rigshospitalet, Denmark. Derived data supporting the findings of this study are available from the corresponding author, LØP, on request.
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2021;71(3):209–249.
- Jakobsen KK, Grønhøj C, Jensen DH, et al. Increasing incidence and survival of head and neck cancers in Denmark: a nation-wide study from 1980 to 2014. Acta Oncol. 2018;57(9):1143–1151.
- Karnov KKS, Grønhøj C, Jensen DH, et al. Increasing incidence and survival in oral cancer: a nationwide Danish study from 1980 to 2014. Acta Oncol. 2017;56(9):1204–1209.
- Van Dijk BAC, Brands MT, Geurts SME, et al. Trends in oral cavity cancer incidence, mortality, survival and treatment in The Netherlands. Int J Cancer. 2016;139(3):574–583.
- Chen SW, Zhang Q, Guo ZM, et al. Trends in clinical features and survival of oral cavity cancer: fifty years of experience with 3,362 consecutive cases from a single institution. CMAR. 2018;ume 10:4523–4535.
- Morton LM, Onel K, Curtis RE, et al. The rising incidence of second cancers: patterns of occurrence and identification of risk factors for children and adults. Am Soc Clin Oncol Educ Book. 2014;2014:e57–67.
- Baxi SS, Pinheiro LC, Patil SM, et al. Causes of death in long-term survivors of head and neck cancer. Cancer. 2014;120(10):1507–1513.
- Fujisawa R, Shibuya H, Harata N, et al. Chronological shifts and changing causes of death after radiotherapy for early-stage oral cancer. Int J Clin Oncol. 2014;19(1):24–29.
- Boakye EA, Buchanan P, Hinyard L, et al. Incidence and risk of second primary malignant neoplasm after a first head and neck squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. 2018;144(8):727–737.
- Chuang SC, Scelo G, Tonita JM, et al. Risk of second primary cancer among patients with head and neck cancers: a pooled analysis of 13 cancer registries. Int J Cancer. 2008;123(10):2390–2396.
- Morris LGT, Sikora AG, Hayes RB, et al. Anatomic sites at elevated risk of second primary cancer after an index head and neck cancer. Cancer Causes Control. 2011;22(5):671–679.
- León X, Venegas MDP, Orús C, et al. Influence of the persistence of tobacco and alcohol use in the appearance of second neoplasm in patients with a head and neck cancer. A case-control study. Cancer Causes Control. 2009;20(5):645–652.
- Day GL, Blot WJ, Shore RE, et al. Second cancers following oral and pharyngeal cancers: role of tobacco and alcohol. J Natl Cancer Inst. 1994;86(2):131–137.
- Shiels MS, Gibson T, Sampson J, et al. Cigarette smoking prior to first cancer and risk of second smoking-associated cancers among survivors of bladder, kidney, head and neck, and stage I lung cancers. J Clin Oncol. 2014;32(35):3989–3995.
- Azad AK, Bairati I, Samson E, et al. Genetic sequence variants and the development of secondary primary cancers in patients with head and neck cancers. Cancer. 2012;118(6):1554–1565.
- Hashibe M, Ritz B, Le AD, et al. Radiotherapy for oral cancer as a risk factor for second primary cancers. Cancer Lett. 2005;220(2):185–195.
- Jensen JS, Jakobsen KK, Mirian C, et al. The Copenhagen oral cavity squamous cell carcinoma database: protocol and report on establishing a comprehensive oral cavity cancer database. Clin Epidemiol. 2019;11:733–741.
- Feller L, Lemmer J. New “second primary” cancers. SADJ. 2012;67(4):175–178.
- Rohde M, Rosenberg T, Pareek M, et al. Definition of locally recurrent head and neck squamous cell carcinoma: a systematic review and proposal for the Odense–Birmingham definition. Eur Arch Otorhinolaryngol. 2020;277(6):1593–1599.
- Ghanizada M, Jakobsen KK, Jensen JS, et al. The impact of comorbidities on survival in oral cancer patients: a population-based, case-control study. Acta Oncol. 2021;60(2):173–179.
- Danish Health Data Authority. The Danish Cancer Registry. Available at: https://www.esundhed.dk/Registre/Cancerregisteret/Nye-kraefttilfaelde. Accesed Jan 22, 2022.
- Min SK, Choi SW, Lim J, et al. Second primary cancers in patients with oral cavity cancer included in the Korea central cancer registry. Oral Oncol. 2019;95:16–28.
- Mroueh R, Nevala A, Haapaniemi A, et al. Risk of second primary cancer in oral squamous cell carcinoma. Head Neck. 2020;42(8):1848–1858.
- Morris LGT, Sikora AG, Patel SG, et al. Second primary cancers after an index head and neck cancer: subsite-specific trends in the era of human papillomavirus-associated oropharyngeal cancer. J Clin Oncol. 2011;29(6):739–746.
- Jacob L, Freyn M, Kalder M, et al. Impact of tobacco smoking on the risk of developing 25 different cancers in the UK: a retrospective study of 422,010 patients followed for up to 30 years. Oncotarget. 2018;9(25):17420–17429.
- Rennemo E, Zätterström U, Boysen M. Impact of second primary tumors on survival in head and neck cancer: an analysis of 2,063 cases. Laryngoscope. 2008;118(8):1350–1356.
- Coca-Pelaz A, Rodrigo JP, Suárez C, et al. The risk of second primary tumors in head and neck cancer: a systematic review. Head Neck. 2020;42(3):456–466.
