Background
The world-wide incidence rate of cutaneous melanoma has been rising continuously with annual increases as high as 4–6% in high-risk fair-skinned populations, and in 2020 nearly 325,000 new patients and 57,000 deaths were reported globally [Citation1]. In Sweden, cutaneous melanoma is the fifth most common malignancy and the incidence is showing an alarming increase with a doubling the last two decades [Citation2]. If detected in an early stage, the disease is curable with surgical resection, while, in the event of advanced disease the prognosis is poor. Until recently the only available systemic treatment was chemotherapy with limited clinical efficacy and a median overall survival (OS) less than one year [Citation3].
Fortunately, the development of immune checkpoint inhibitors (ICI) and targeted therapies with BRAF and MEK inhibitors have vastly improved the outcome of patients with metastatic melanoma [Citation4–9]. Several clinical studies have demonstrated that programmed death receptor 1 (PD-1) inhibitors, as monotherapy or in combination with the cytotoxic T-lymphocyte associated protein 4 (CTLA-4) inhibitor ipilimumab, are efficient in treating metastatic melanoma [Citation5,Citation7,Citation10]. There is also evidence supporting ICIs as the first line of treatment in patients with BRAF mutated melanoma [Citation11]. Treatments with PD-1 inhibitors generates a response in more than 40% of melanoma patients regardless of their BRAF mutation status. For patients who respond, the median duration of the response is several years, and some patients are potentially cured [Citation12]. However, within the first year nearly 50% of the patients suffer from progressive disease [Citation5,Citation13,Citation14]. Progressing patients with BRAF mutated melanoma are candidates for treatment with BRAF and MEK inhibitors, to which the majority respond, but the duration of response is limited to a median of approximately 1.5 years [Citation15]. Patients with primary or secondary resistance to ICIs are hence left with inadequate treatment options and further research is of essence [Citation5,Citation13].
Radiotherapy (RT) is currently mainly used as a symptom-relieving palliative measure in metastatic melanoma patients. Based on early in vitro and retrospective studies, melanoma was considered a RT resilient tumor [Citation16,Citation17]. This resistance to RT is believed to stem from different features often present in melanoma cells, including resistance to apoptosis, high DNA repair capacity, high proliferation capacity and poor cell differentiation, in addition to a hypoxic tumor microenvironment [Citation17].
Stereotactic body radiation therapy (SBRT) is today a recognized RT-technique in which the RT is delivered with inhomogeneous dose distribution in a few fractions and with high absorbed doses per fraction (usually 10–20 Gy) with resultant high biological effectiveness. SBRT has been successful thanks to the high rate of local control of the treated lesions and the limited side effects for the patients [Citation18]. Even melanoma tumors with their relative resistance to RT have a local control rate with SBRT that is over 80% [Citation19]. Retrospective studies in metastatic melanoma patients have shown that RT, conventional and hypo-fractionated RT as well as SBRT, in combination with ICIs, is well tolerated and the type and grade of side effects are in a range that could be expected for each of the modalities separately [Citation17,Citation19–25].
Radiation of tumors can evoke an immune response through various processes, including apoptosis, DNA damage, tumor lysis, release of tumor antigens and cytokines, inflammation, and infiltration of immune cells, including tumor-specific cytotoxic CD8+ T-cells which enhances the diversity of the T-cell receptor (TCR) repertoire of intertumoral T-cells [Citation26–28]. RT induced immune activation in the target area can potentially result in a systemic activation and an immune mediated shrinkage also of tumors distant to the irradiated area – a phenomenon known as an ‘abscopal effect’ [Citation29,Citation30]. An abscopal effect is, however, an event rarely seen when RT is given solely [Citation31].
An attractive approach would thus be to use the radiation induced immune response to potentiate the clinical effect of ICI and further enhance tumor shrinkage outside of the irradiation field. In retrospective reports of melanoma patients receiving RT on progressing metastases while continuing treatment with PD-1 inhibitors, an abscopal shrinkage has been observed in 15–35% of the non-irradiated tumor lesions [Citation21,Citation23]. However, prospective studies are lacking [Citation21,Citation23,Citation32]. There is moreover no definite consensus on the optimal dose per fraction nor the total absorbed tumor dose for triggering an abscopal effect [Citation33]. Some experts argue in favor of a conventional fractionated dosage [Citation34] whereas others believe that a high-dose-hypofractionated radiation dose shows improved response [Citation35,Citation36]. The PROMMEL phase II trial aims to study the hypothesis that an abscopal effect can be generated when adding SBRT to ICI resistant melanoma metastases while continuing treatment with ICIs. If our hypothesis is supported, it will be a highly anticipated treatment method for this patient group where efficient treatment options are lacking. Additionally, biomarkers in blood and tumors samples will be assessed to study the biologic processes and mechanisms behind abscopal effect.
Methods
Aim
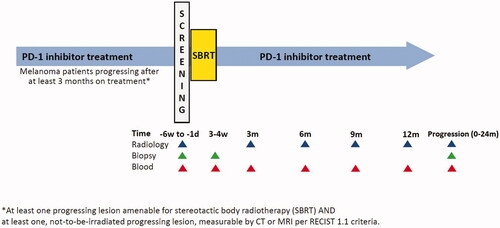
This is to our knowledge the first prospective trial focusing on melanoma patients and the occurrence of abscopal effect when ICI resistant metastases are treated with SBRT technique. The PROMMEL study is a phase II open-label multicenter trial supported by the Swedish Melanoma Study Group (SMSG). See for overall study design.
Study population
The study population includes metastatic melanoma patients progressing on PD-1 inhibitor treatment, meeting all the inclusion and exclusion criteria (see below). Patients in this study will be recruited from melanoma oncology clinics at three Swedish University Hospitals: Karolinska University Hospital in Stockholm, Sahlgrenska University Hospital in Gothenburg and Uppsala University Hospital.
Inclusion criteria
Age ≥ 18.
ECOG performance status of 0–1.
Signed and dated written informed consent.
Verified disease progression after at least 3 months of PD-1 inhibitor treatment (alone or in combination with CTLA-4 inhibitor) as the first line of therapy for unresectable metastatic cutaneous melanoma. Patients with primary or secondary resistance to the PD-1 inhibitor treatment will be included.
At least one progressing metastasis that is judged to be safely amenable to SBRT in the opinion of treating radiation oncologist. A fine-needle aspiration (FNA) biopsy from a progressing lesion or a new lesion is recommended to confirm the presence of viable tumor cells.
At least one, not-to-be-irradiated new or progressing lesion measurable as assessed by CT or MRI per RECIST 1.1 criteria where up to four radiation target fields are allowed.
No contraindication for continuing PD-1 inhibitor 12 months after radiation.
Exclusion criteria
Inability to understand given information or undergo study procedure according to protocol.
Pregnant or breast-feeding. Patients must agree to safe contraception.
Systemic treatment with either corticosteroids (> 10 mg daily prednisone equivalents) or other immunosuppressive medication.
Have an active infection requiring systemic therapy.
Concomitant therapy with any anti-tumor medication other than ICI.
Prior RT preventing the study intervention with SBRT.
Location, size, or number of metastases deemed too large or not appropriate for SBRT.
Active brain metastases.
Prior malignancy within five years.
Endpoints
The primary endpoint is to evaluate overall response rate (ORR) in non-irradiated lesions according to RECIST 1.1 (37). The secondary endpoints are ORR in irradiated lesions, median progression-free survival (PFS) and OS, PFS and OS rate at 6 and 12 months and adverse events. Tumor biopsies, primarily from non-irradiated lesions, will be secured for translational research, including genomic (DNA and RNA) and proteomic analysis exploring tumor heterogeneity and mechanisms of ICI resistance. Blood samples for biomarker analysis, including FACS analyses of peripheral leukocytes, analyses of plasma cytokines, extracellular vesicles/exosomes and circulating tumor DNA (ctDNA), will be taken at both screening and at follow-up visits. Secondary and exploratory endpoints can be found specified in detail in the Study protocol attached in the Supplementary Material.
Study design
The study participant will undergo SBRT and continue the same regimen of anti PD-1 inhibitor therapy (nivolumab or pembrolizumab) until progression, discontinue due to side effects or up to 12 months of treatment. Assessment whether at least one metastasis is suitable for SBRT is made by the treating radiation oncologist. Overview of fractionation schedules for different target locations are shown in . Target areas are extracranial metastases, such as (but not limited to) lungs, lymph nodes, liver, and bone. A CT or PET-CT scan will be performed every three months to evaluate clinical response according to RECIST 1.1 criteria [Citation38]. The subjects will be followed until study discontinuation, death, disease progression or up to a maximum of 24 months.
Table 1. Overview of fractionation schedules, target volume definition and dosage for different target locations possible to use in the study.
The study will be conducted according to Simon’s two stage minimax design [Citation39]. Patients will be enrolled in two stages, the first consisting of 13 patients. If no objective responses are reported in any of the patients in this step, the study is interrupted early for futility. If objective response in non-irradiated lesions has been achieved in at least one patient when the 13th patient has been evaluated, the recruitment can proceed into the second step. Fourteen more patients will then be included up to a total of 27 patients to determine ORR in non-irradiated lesions along with the 95% confidence interval. The sample size and power estimation are based on the primary endpoint ORR. Power is required to be 80%. We consider ORR of 15% to be sufficient to conclude that the treatment combination is useful. A low number is motivated by the fact that patients who have experienced disease progression, are unlikely to respond if continuing with the same treatment only.
‘Pseudo-progression’ is a phenomenon that has been described in association with ICI treatment, where an increase in tumor size or occurrence of a new lesion is related to inflammation and infiltration of immune cells, and not to progression of tumor cells. In melanoma, pseudo-progression is expected to occur in around 5% of ICI treated patients [Citation40,Citation41]. In the PROMMEL study, the following steps are taken to decrease the likelihood of including pseudo-progressing patients:
The lesion(s) should increase with at least 30% in size, which is larger than the RECIST 1.1. criteria of 20% increase for progressive disease
Biopsy is recommended from target lesions to confirm if tumors are dominated by viable melanoma cells.
Radiologic evaluation is performed by radiologists with expertise in evaluating response in ICI treated melanoma patients and familiar with the specific patterns associated with pseudo-progression, including mixed responses.
Ethical consideration
Both ICI and SBRT are well-established treatments that have shown to be effective in melanoma patients, and data from retrospective studies when the treatments have been combined have not shown more side-effects, than what could be expected from each of these therapies separately. The treatment combination with SBRT and ICI in the PROMMEL study is hence considered ethically justified and potential side effects will be closely monitored and reported. The investigators will ensure that all subjects are given oral and written information regarding risks of participating and the opportunity to discontinue from the study at any time. All subjects are required to give signed and dated informed consent for participation. The study will be performed according to Good Clinical Practice (GCP) guidelines and the Declaration of Helsinki. The trial protocol is reported in accordance with the SPIRIT guidelines [Citation42] for clinical trials please see Supplementary Material. The study has been approved by Swedish Ethical Review Authority (Dnr 2020-05584) and by the Swedish Biobank Authority. ClinicalTrials.gov identifier NCT04793737, registered on March 11th, 2021.
Perspectives
In the PROMMEL trial, metastatic melanoma patients progressing on ICI, will be treated with SBRT of their tumors and continue treatment with PD-1 inhibitors. We aim to study the hypothesis that this treatment strategy will create an abscopal effect in nonirradiated tumor(s). Since this study is the first prospective trial focusing on melanoma patients and abscopal effect, we anticipate that the result will be of high interest whether the study outcome supports or disproves the hypothesis.
Trial status
As of September 13th, 2021, the PROMMEL study is open at the three participating sites, in Stockholm, Gothenburg and Uppsala. On February 25th, 2022, six patients have been included and all of them have received the SBRT intervention and thereafter continued with the PD-1 inhibitor treatment.
Authors contributions
HH is the principal investigator and contributed with the design of the study and writing of the study protocol. VG, RL, GM, KK, RPZ, KV, ROB, GU, KL, and LN contributed to the study design and protocol. HH, EB, LN, GU, JF, HE, BJ and KH are responsible for recruitment and follow-up of the patients. VG, UI, CR, EA, KK, RPZ, PW, and KL are responsible for radiologic evaluation and planning of SBRT. HH, MY, UE, SW, SEB, RK, KV, BF, and EDR are responsible for collection and analyses of the patients’ biological samples. EB and HH drafted the manuscript that all authors have read, participated in revisions, and approved the final manuscript.
Supplemental Material
Download PDF (1.1 MB)Supplemental Material
Download PDF (141.3 KB)Supplemental Material
Download MS Word (124 KB)Acknowledgements
We are grateful for the financial support from the funders. The funding bodies had no part in the design of the study and will have no part in the analysis or interpretation of data, in writing the manuscript or in the decision to submit the manuscript for publication.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability
Data sharing is not applicable to this study protocol since no data were generated or analyzed. Results from the PROMMEL study will be published and specific data will be available on reasonable request.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel R, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2021;71(3):209–249.
- Socialstyrelsen. Statistics on Cancer Incidence 2020 Socialstyrelsen: Socialstyrelsen; 2021. [Available from: www.socialstyrelsen.se/statistik-och-data/statistik/statistikamnen/cancer/.
- Middleton MR, Grob JJ, Aaronson N, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol. 2000;18(1):158–166.
- Robert C, Grob JJ, Stroyakovskiy D, et al. Five-Year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med. 2019;381(7):626–636.
- Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–2532.
- Dummer R, Ascierto PA, Gogas HJ, et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19(5):603–615.
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330.
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954.
- Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371(20):1877–1888.
- Hamid O, Robert C, Daud A, et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol. 2019;30(4):582–588.
- Atkins M, Lee S, Chmielowski B, et al. DREAMseq (doublet, randomized evaluation in advanced melanoma sequencing): a phase III trial—ECOG-ACRIN EA6134. J Clin Oncol. 2021;39(36_suppl):356154–356154.
- Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Long-Term outcomes with nivolumab plus ipilimumab or nivolumab alone versus ipilimumab in patients with advanced melanoma. J Clin Oncol. 2022;40(2):127–137.
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-Year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381(16):1535–1546.
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34.
- Ascierto PA, Dummer R, Gogas HJ, et al. Update on tolerability and overall survival in COLUMBUS: landmark analysis of a randomised phase 3 trial of encorafenib plus binimetinib vs vemurafenib or encorafenib in patients with BRAF V600-mutant melanoma. Eur J Cancer. 2020;126:33–44.
- Mahadevan A, Patel VL, Dagoglu N. Radiation therapy in the management of malignant melanoma. Oncology. 2015;29(10):743–751.
- Rogers SJ, Puric E, Eberle B, et al. Radiotherapy for melanoma: More than DNA damage. Dermatol Res Pract. 2019;2019:9435389.
- Song CW, Kim MS, Cho LC, et al. Radiobiological basis of SBRT and SRS. Int J Clin Oncol. 2014;19(4):570–578.
- Franceschini D, Franzese C, De Rose F, et al. Role of extra cranial stereotactic body radiation therapy in the management of stage IV melanoma. Br J Radiol. 2017;90(1077):20170257.
- Grimaldi AM, Simeone E, Giannarelli D, et al. Abscopal effects of radiotherapy on advanced melanoma patients who progressed after ipilimumab immunotherapy. Oncoimmunology. 2014;3:e28780.
- Liniker E, Menzies AM, Kong BY, et al. Activity and safety of radiotherapy with anti-PD-1 drug therapy in patients with metastatic melanoma. Oncoimmunology. 2016;5(9):e1214788.
- Aboudaram A, Modesto A, Chaltiel L, et al. Concurrent radiotherapy for patients with metastatic melanoma and receiving anti-programmed-death 1 therapy: a safe and effective combination. Melanoma Res. 2017;27(5):485–491.
- Funck-Brentano E, Baghad B, Fort M, et al. Efficacy of late concurrent hypofractionated radiotherapy in advanced melanoma patients failing anti-PD-1 monotherapy. Int J Cancer. 2020;147(6):1707–1714.
- Seegenschmiedt MH, Keilholz L, Altendorf-Hofmann A, et al. Palliative radiotherapy for recurrent and metastatic malignant melanoma: prognostic factors for tumor response and long-term outcome: a 20-year experience. Int J Radiat Oncol Biol Phys. 1999;44(3):607–618.
- Chicas-Sett R, Morales-Orue I, Castilla-Martinez J, et al. Stereotactic ablative radiotherapy combined with immune checkpoint inhibitors reboots the immune response assisted by immunotherapy in metastatic lung cancer: a systematic review. Int J Mol Sci. 2019;20(9):2173.
- Eriksson D, Stigbrand T. Radiation-induced cell death mechanisms. Tumour Biol. 2010;31(4):363–372.
- Lugade AA, Sorensen EW, Gerber SA, et al. Radiation-induced IFN-gamma production within the tumor microenvironment influences antitumor immunity. J Immunol. 2008;180(5):3132–3139.
- Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520(7547):373–377.
- Brooks ED, Chang JY. Time to abandon single-site irradiation for inducing abscopal effects. Nat Rev Clin Oncol. 2019;16(2):123–135.
- Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58(3):862–870.
- Abuodeh Y, Venkat P, Kim S. Systematic review of case reports on the abscopal effect. Curr Probl Cancer. 2016;40(1):25–37.
- Roger A, Finet A, Boru B, et al. Efficacy of combined hypo-fractionated radiotherapy and anti-PD-1 monotherapy in difficult-to-treat advanced melanoma patients. Oncoimmunology. 2018;7(7):e1442166.
- Wang D, Zhang X, Gao Y, et al. Research progress and existing problems for abscopal effect. Cancer Manag Res. 2020;12:6695–6706.
- Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15(17):5379–5388.
- Filatenkov A, Baker J, Mueller AM, et al. Ablative tumor radiation can change the tumor immune cell microenvironment to induce durable complete remissions. Clin Cancer Res. 2015;21(16):3727–3739.
- Wang SJ, Jhawar SR, Rivera-Nunez Z, et al. The association of radiation dose-fractionation and immunotherapy use with overall survival in metastatic melanoma patients. Cureus. 2020;12(6):e8767.
- Cox BW, Spratt DE, Lovelock M, et al. International spine radiosurgery consortium consensus guidelines for target volume definition in spinal stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2012;83(5):e597–e605.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247.
- Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10(1):1–10.
- Basler L, Gabryś HS, Hogan SA, et al. Radiomics, tumor volume, and blood biomarkers for early prediction of pseudoprogression in patients with metastatic melanoma treated with immune checkpoint inhibition. Clin Cancer Res. 2020;26(16):4414–4425.
- Lee JH, Long GV, Menzies AM, et al. Association between circulating tumor DNA and pseudoprogression in patients with metastatic melanoma treated with anti-Programmed cell death 1 antibodies. JAMA Oncol. 2018;4(5):717–721.
- Chan AW, Tetzlaff JM, Gotzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586.