Abstract
Background
Early diagnosis and compression treatment are important to prevent progression in breast cancer-related arm lymphedema (BCRAL). However, some mild BCRAL can be reversible, and therefore, compression treatment may not be needed. The aim of this study was to investigate the proportion of women with mild BCRAL showing progression/no progression of lymphedema after treatment with or without compression garments, differences in changes of lymphedema relative volume (LRV), local tissue water and subjective symptoms during 6 months. Also, adherence to self-care was examined.
Material and methods
Seventy-five women diagnosed with mild BCRAL were randomized to a compression group (CG) or noncompression group (NCG). Both groups received self-care instructions, and the CG were treated with a standard compression garment (ccl 1). Women in the NCG who progressed in LRV ≥2%, or exceeded 10% dropped out, and received appropriate treatment. The proportion showing progression/no progression of LRV, and changes in LRV was measured by Water Displacement Method. Changes in local tissue water were measured by Tissue Dielectric Constant (TDC), subjective symptoms by Visual Analogue Scale, and self-care by a questionnaire.
Results
A smaller proportion of LRV progression was found in the CG compared to the NCG at 1, 2 and 6 months follow-up (p ≤ 0.013). At 6 months, 16% had progression of LRV in the CG, compared to 57% in the NCG, (p = 0.001). Thus, 43% in the NCG showed no progression and could manage without compression. Also, CG had a larger reduction in LRV, at all time-points (p ≤ 0.005), and in the highest TDC ratio, when same site followed, at 6 months (p = 0.025). Subjective symptoms did not differ between the groups, except at 1 month, where the CG experienced more reduced tension (p = 0.008). There were no differences in adherence to self-care.
Conclusion
Early treatment with compression garment can prevent progression in mild BCRAL. Trial registration: ISRCT nr ISRCTN51918431
Background
Breast cancer-related arm lymphedema (BCRAL) is primarily caused by axillary surgery and radiotherapy [Citation1]. The incidence of BCRAL varies depending on the diagnostic method and thresholds used. In a Swedish cohort of 292 women treated with axillary lymph node dissection (ALND) and radiotherapy, the incidence of BCRAL was 38.7%, when a threshold value of lymphedema relative volume (LRV) ≥5% was used [Citation2]. However, in a systematic review with higher thresholds used, the estimated incidence was calculated to be 21.4% [Citation1]. BCRAL is considered a chronic condition and without treatment, progression can be expected [Citation3]. The standard treatment of BCRAL includes using a compression garment and recommended self-care [Citation4].
Some studies of patients with BCRAL treated with ALND have shown that early diagnosis and intervention, including compression treatment is important to prevent progression [Citation5–8] and some studies indicate that it is the edema volume at the start of treatment that is the most important predictive factor [Citation2,Citation9,Citation10]. Although early diagnosis and treatment of BCRAL are recommended [Citation11], there is a lack of knowledge about the proportion of women who do not progress in lymphedema. Also, very few studies have assessed the effect of compression garments in women with BCRAL [Citation12]. In a study on early diagnosis (LRV ≥5%) and treatment, it was shown that 27% had a LRV ≤5%, on average 5 years after diagnosis [Citation2]. However, it was unclear if the maintenance of the low level of arm volume, was due to the treatment with compression garment or if the edema had otherwise stabilized at a low level. An edema of LRV ≥5–10% are associated with progression [Citation13–15] and therefore recommended as a threshold for intervention [Citation11]. However, in mild BCRAL, the lymphedema can be local [Citation16,Citation17] and thus, new methods to detect mild BCRAL are needed. Currently, there is no consensus about the best method to diagnose mild BCRAL. The Bioimpedance Spectroscopy (BIS) method can identify early BCRAL [Citation18]. However, the Tissue Dielectric Constant (TDC) is an easy new method to measure local tissue water and has been used together with palpation and the Water Displacement Method (WDM) for diagnosis of mild BCRAL [Citation19,Citation20]. TDC has shown a higher sensitivity than the BIS method and may therefore be better in assessing early BCRAL [Citation20].
Subjective symptoms of tension and heaviness have been used as diagnostic criteria for lymphedema [Citation10,Citation20,Citation21], especially in women with moderate/severe lymphedema and decreased symptoms is an important treatment goal. Therefore, it is important to examine subjective symptoms during treatment with and without a compression garment, in mild BCRAL.
As lymphedema often could become a chronic condition, lifelong compression treatment may be needed, which entails a cost for the patient and the health care system. Even more important is that wearing a compression arm sleeve has been described as a major problem by the patients [Citation22]. Therefore, it is important to examine the proportion of patients that can manage without compression and the efficacy of early compression treatment. The aim of this study was to investigate (i) the proportion of women with mild BCRAL showing progression/no progression of lymphedema after treatment with or without a compression garment, and (ii) differences in changes of LRV, local tissue water and subjective symptoms during six months. Adherence to the self-care regime was also examined for all participants.
Material and methods
Study design
This is a prospective randomized controlled trial (RCT), where women with BCRAL were randomized to use of a compression garment or not and were followed for six months. The CONSORT checklist for RCTs was followed and the study was registered in ISRCTN51918431.
Participants
Women treated for unilateral breast cancer, with ALND and diagnosed with mild BCRAL at the Lymphedema Unit, Skåne University Hospital and at the Physiotherapy Cancer Unit, Karolinska University Hospital, were potential participants in the study. Inclusion criteria were: mild BCRAL defined as increased skin and subcutis thickness [Citation23] compared to the non-affected arm [Citation2,Citation19,Citation20], in addition to either a threshold TDC ratio (≥1.45 in the upper arm, and/or ≥1.3 in the forearm) [Citation24], and/or LRV ≥5–≤8% [Citation13,Citation14]. Exclusion criteria were: recurrent cancer, concurrent diseases, cognitive disability or unable to understand or speak Swedish.
Procedures
The women were called for routine clinical follow-ups 4 to 6 weeks after surgery and 3 to 4 months after completed radiotherapy [Citation25]. They were also informed to return if lymphedema was noticed at any time. When mild BCRAL was diagnosed, those who agreed to participate were included in the study and were called for visits after 1, 2, 3 and 6 months, corresponding to (T1, T2, T3, T6) from baseline. The participants were consequently randomized to a compression group (CG) or noncompression group (NCG), by picking a sealed envelope, in which the group allocation was stated. The randomization was done in blocks of 10 (allocation ratio of 1:1). The randomization, examinations, and treatment were carried out by two of the authors (KJ, KB) and by two other experienced lymphedema therapists (CS, PN), unblinded to the group allocation and to measurements at previous time-points. Measurements of arm volume, local tissue water, subjective symptoms, weight (kg), and garment pressure were performed at all time-points. At baseline, height (in meters), was measured, data about surgical methods and adjuvant treatments were retrieved from medical records and the participants responded to a study-specific questionnaire regarding age, education, and marital status. At T6, the participants rated their physical activity/exercise/housework and adherence to recommended self-care.
Ethical approval
The Regional Ethical Board, Lund University approved the study, D nr:2014/399. All participants provided informed consent to participate in the RCT. Data were collected from September 2014 to April 2019.
Outcomes
Primary outcome was the proportion of participants, showing progression/no progression of LRV at T1, T2, T3, T6. Secondary outcomes were differences in changes of LRV, local tissue water, subjective symptoms at T1, T2, T3, T6, and adherence to recommended self-care at T6.
Clinical measurements
Arm volume
Arm volume of both arms was measured by WDM. The straight arm was lowered into a container filled with water and the closed fist placed on the container floor. LRV values were obtained by calculating the edema volume divided by the total arm volume of the nonaffected arm and were adjusted for hand dominance by ±1.5% [Citation26]. WDM has shown good reliability with an intraclass coefficient (ICC) of 0.99, measured in the same way as in our study, in patients with lymphedema in the upper extremity [Citation27]. Progression was defined as an increase of LRV ≥2% compared to baseline or if exceeded LRV >10%, based on calculated standard error of measurement (SEM) of 0.1 and minimal detectable change (MDC) of 1.0% [Citation28].
Local tissue water
Local tissue water was measured by the MoistureMeterD or MoistureMeterD compact, (Delfin Technologies Ltd, Finland). A low intensity 300 MHz signal is transmitted from the probe in contact with the skin, and TDC value is calculated. The probe has a penetration depth of 2.5 mm and corresponds to maximum 78.5% of pure water content. Six points were measured once [Citation29], 5 cm proximal and 5 cm distal to the antecubital fossa (medial, frontal, and lateral). If lymphedema was palpated more proximally or distally in the arm, complementary measurements were taken 15 cm proximal or distal to the antecubital fossa. Mayrovitz et al. have reported the TDC method to be reliable with an ICC value of 0.77, a SEM of 0.03 and a MDC of 0.08 in healthy persons [Citation30].
Subjective symptoms
The participants self-rated experiences of heaviness, tension and pain in the affected arm were rated on a horizontal 100 mm visual analogue scale (VAS) [Citation31].
Self-care
Self-care data were collected using a questionnaire concerning physical activity level/exercise and housework during the last four weeks on a six-point scale (from sedentary to high physical activity) [Citation32]. Frequency of self-massage was rated on a four-point scale (no massage, seldom, two–three times a week, every day), and use of compression garment on a three-point scale (not at all, half the day or the whole day).
Garment pressure
The garment pressure in the upper arm was measured using an air-filled pressure transducer (Kikuhine, TT Meditade, Sörö, Denmark).
Interventions
The CG received circular knitted compression sleeves (ccl 1) or if needed, individually adjusted compression sleeves (ccl 2) for daily wearing during six months, and counseling in self-care about exercise, weight control, skin care and instructions in self-massage. The self-massage comprised instructions on light strokes over the shoulder and arm in a proximal direction for about 10–15 min a day. If the self-massage was perceived as effective, participants were encouraged to continue, otherwise to stop. The NCG received instructions in self-care only. Due to ethical reasons, the participants in the NCG who increased in LRV ≥2% from baseline dropped out from their group allocation and started to wear compression garments. If LRV exceeded ≥10% the participants from both groups dropped out and received extended treatment.
Statistical power and analysis
A power calculation with a power of 0.85 at p ≤ 0.05 level of significance showed that 80 participants should be included to be able to detect an arm volume difference of 20% between the groups. However, we ceased recruitment when 75 participants had been included due to an unreasonably time-consuming recruitment and inclusion process. Descriptive statistics were presented as mean ± SD for continuous data and as number and proportion (in %) for categorical data. Differences between groups were calculated with Pearson Chi square test for nominal data, Mann–Whitney test for ordinal data and t-test for continuous data. The TDC data were not normally distributed and therefore presented as both median (min-max) and mean ± SD, to be able to compare results with other studies. The participants in the NCG who progressed in LRV ≥2%, and those in both groups that exceeded 10% were defined as having progression and the remaining as having no progression. They were not included in the LRV analysis, local tissue water, subjective symptoms, and self-care after the time-point when they progressed. However, post hoc analyses of LRV and local tissue water of the participants who progressed in LRV ≥2%, were performed and presented. The analyses were carried out in IBM SPSS Statistics 26 and a significance level of p < 0.05 (two tailed) was chosen.
Results
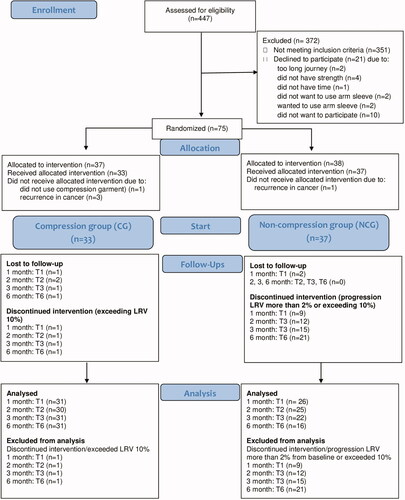
A total of 447 women following surgery to the breast and axilla, were called for follow up visits, and 96 women were diagnosed with mild BCRAL. Of these, 21 declined to participate. The 75 women who agreed to participate, were randomized to a CG or a NCG. During the trial, five participants were excluded, mainly due to recurrence in the cancer disease. The remaining 70 were eligible for data analysis, 33 in the CG and 37 in the NCG (). The participants in the CG and the NCG were comparable in baseline data, except for surgery. In the CG, 61% had underwent surgery with mastectomy, compared to 35% in the NCG (p = 0.033), (). Of the 33 participants in the CG, 29 were treated with compression sleeves (ccl 1) and four with individual adjusted sleeves (ccl 2). In nine, the hand was also affected, and compression gloves were therefore prescribed. Pressure under the garment at T6 was on average 11.2 ± 2.8 mmHg.
Table 1. Participant characteristics of women with mild breast cancer related arm lymphedema, in the compression group (CG) and noncompression group (NCG), (n = 70).
Outcomes
Proportions of participants who progressed/did not progress in LRV
A significantly smaller proportion in the CG exceeded LRV ≥2% from baseline or exceeded 10%, compared to the NCG. The difference at T1 was 3% vs 26%, (p = 0.005), at T2, 6% vs 32%, (p = 0.013) and at T6, 16% vs 57%, (p = 0.001). At T6, 84% in the CG, showed no progression in LRV compared to 43% in the NCG ().
Table 2. Proportion of participants with no progression/ progression of lymphedema relative volume (LRV) over 6 months in the compression group (CG) and noncompression group (NCG), n = 70.
Lymphedema relative volume (LRV)
The CG had lower LRV, compared to the NCG at T2 (p < 0.001), T3 (p = 0.001) and T6 (p < 0.001), (). The CG also had a larger decrease in LRV at all time points. The mean difference between the CG and the NCG in changes at T1 was −1.9% vs +0.3%, (p = 0.005), at T2, −2.6% vs +0.1%, (p = 0.001), at T3, −2.6% vs −0.1%, (p = 0.001), and at T6, −3.8% vs +0.1, (p < 0.001), ().
Table 3. Lymphedema relative volume (LRV) measured with water displacement method over six months in the compression group (CG), and noncompression group (NCG), (n = 70).
Local tissue water
When the same site was followed, the CG had a larger decrease in the highest TDC ratio at T6, compared to the NCG, with a median change of −0.28 vs −0.10 (p = 0.025), (). The CG also had a larger decrease, close to significant, compared to the NCG, at T2, (p = 0.059), and at T3, (p = 0.053). Also, when the highest TDC ratio, by any site was followed, the CG had a larger decrease than the NCG, and close to significance at T6 (p = 0.052), ().
Table 4. Local tissue water measured with Tissue Dielectric Constant (TDC) over 6 months in the compression group (CG), and noncompression group (NCG), (n = 70).
Subjective symptoms
Self-rated feeling of tension, heaviness and pain, were low at all time points (median 0) in both groups, with no significant differences between the CG and NCG at any time-point (data not presented). However, there was a significant difference in change of tension, at T1, where the CG decreased more than the NCG, median difference (min-max): 0 (−56 to 29) vs 0 (−45 to 39), (p = 0.008).
Adherence to recommended self-care
At T6, all 33 women in the CG rated that they wore the compression garment; 93% the whole day, and 7% half the day. There were no significant differences between CG and NCG in self-rated frequency of physical activity/exercise and housework, or frequency of performed self-massage at T6 (data not presented).
Participants with progression in LRV ≥2% from baseline
In the CG, 4(13%) had progressed, both at T3 and T6 (). At baseline, LRV was mean (SD) −1.3(2.7)% and at the time of progression, 2.0(2.7)%. At baseline, the highest TDC ratio, was mean (SD) 1.66(0.10). At the time of progression, the highest TDC ratio followed by any site, was mean (SD) 1.44(0.28). In the NCG, 12(32%) had progressed at T3 and 17(46%) at T6 (). At baseline, LRV was mean (SD) 2.9(3.7)%. At the time of progression, LRV was mean(SD) 6.5(3.4)% and after 6 months with compression, 4.0(6.4)%. At baseline, the highest TDC ratio was mean (SD) 1.53(0.24). At the time of progression, the highest TDC ratio followed by any site was 1.40(0.29) and after 6 months with compression, 1.43(0.32).
One participant (3%) in the CG exceeded LRV 10% at 1 month and 3(8%) in the NCG exceeded LRV 10% at T3 and 4(11%) at T6 ().
Discussion
In the present study, we evaluated the effect of compression garment versus no compression following mild BCRAL for six months. We found that a smaller proportion in the CG showed progression of LRV, than in the NCG. Also, the CG had a larger decrease in LRV at all time points and in the highest TDC ratio, at T6. However, 43% in the NCG did not have progression of LRV and could manage without compression. Subjective symptoms in the arm were generally low and did not differ between the groups, except at T1, where the CG had more decrease in tension. Adherence to self-care was similar in both groups. These findings show that early treatment with a compression garment can prevent progression in mild BCRAL.
There were significantly fewer participants who showed progression of LRV in the CG at T1, T2, and T6, compared to the NCG. In the CG, 16% progressed in LRV ≥2% from baseline or exceeded 10%, compared to 57% in the NCG. These results show that wearing compression garment is important and effective to prevent progression in mild BCRAL. Among all the participants who had progression of LRV, 77% had progression already within three months from baseline/diagnosis. This emphasizes the importance of follow-up visits close to lymphedema diagnosis, which have also been reported by others [Citation13,Citation14]. Furthermore, we found that 43% of the participants in the NCG had no progression of LRV, and these women could manage without a compression garment. This is an important result that should be considered in all research of early mild BCRAL, as well as in clinical settings. A previous study has found that preventive use of a compression garment after ALND can decrease the incidence of arm lymphedema with 10% [Citation33]. The result in our study indicates that preventive use of compression garment for all patients treated with ALND can be questioned and will put an unnecessary burden on the patient and a higher cost for both the patient and the health care system. Regression of BCRAL has been noted earlier. Kilgore et al diagnosed BCRAL with BIS and treated women with compression arm sleeves in 4 weeks (follow-up time, mean 21 months), and found that 82% of these returned to baseline measurements [Citation7]. However, randomized studies reporting the effect of compression treatment in mild BCRAL are lacking. Thus, the results in our study contribute to increased knowledge about progression/no progression, but do not identify which patients who can manage without compression.
There were significant differences in decrease of LRV in favor of the CG, although more participants in the CG had undergone a mastectomy, which is a known risk factor for lymphedema, and participants who progressed in NCG were excluded from the analysis. The CG decreased, on average −1.9%, already at T1, indicating that the use of compression garment had a fast impact on LRV. Furthermore, the participants in the CG continued to decrease in LRV until T6 (mean difference −3.8%). Our findings are larger than the SEM of 0.1% and MDC of 1.0% [Citation28], and therefore, we consider the decrease of LRV in our study as clinically relevant. Our results are in line with the study by Stout-Gergich et al. who evaluated treatment with a compression arm sleeve, ccl 2, for 4 weeks and found a mean arm volume decrease of −2.4%, on average 4.6 months from start of intervention [Citation34]. The results in our study showed that it is beneficial to use a compression garment during 6 months. However, future studies are needed to examine the long-term results and whether early treatment can decrease the development of persistent irreversible lymphedema.
We also found a significant difference in change of the highest TDC ratio between the groups at T6 when the same site was followed. The CG decreased median −0.28, compared to −0.10 in the NCG, which we consider as a clinically relevant decrease as the MDC is 0.08 [Citation30]. Also, at T2 and T3 the differences in change were close to significant between the groups. This indicates that the compression garment has an impact on local tissue water. However, we may have missed significant results at the other time-points, due to exclusion of those with lymphedema progression. Larger prospective studies are needed to examine the relationship between local tissue water and progression of arm volume.
The present study showed that self-rated subjective symptoms in the arm were generally low at all time-points and did not differ between the groups. Our results are consistent with previous research showing a correlation between perceived symptoms and the degree of lymphedema volume [Citation10]. Regular examinations of women at risk are therefore even more important, because women with mild BCRAL do not necessarily have subjective symptoms in the arm and may not be attentive to onset of lymphedema. However, a significantly larger reduction of tension was found in the CG compared to the NCG at T1. This indicates that use of compression garment may have a positive effect on perceived tension in the arm.
Strengths and limitations
Strengths with the present study were the design with several follow-up visits during six months, and access to data of rated self-care. There are also some limitations. The study was registered in ISRCTN retrospectively and the assessors were not blinded to group allocation which may have affected the results. There is no consensus on how to diagnose mild BCRAL and our results depend on our definition of mild BCRAL and the thresholds used for WDM and TDC. However, the inclusion criteria of LRV ≥5% is recommended for early diagnosis and the TDC thresholds +3 SD of mean have probably reduced the risk of false-positive lymphedema. We had no possibility to measure the arm volume preoperatively, and therefore, some participants may have been misclassified due to arm asymmetry. However, palpation of increased skin and subcutis thickness was an inclusion criterion and performed before the WDM and TDC measurements. Furthermore, a lymphedema is expected to progress without treatment. Thus, we had to, on ethical grounds, give treatment to participants in the NCG who progressed in LRV and therefore they could not be included in analyzes of LRV, TDC and subjective symptoms after the time-point of progression.
Conclusion
We found that early treatment with a compression garment can prevent progression in mild BCRAL, showing smaller proportions of progression, larger reduction in LRV and local tissue water, and reduced experience of tension, compared to no compression garment. However, 43% in the NCG did not show progression and could manage without compression. Future studies are needed to examine the long-term effects, and factors influencing progression of mild BCRAL.
Acknowledgements
The authors would like to thank the participants in this study, colleagues RPT Christina Snöbohm and Polymnia Nikolaidis who have assisted in the examinations and PhD Michael Miller for language editing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that supports the findings of this study are available on request from the corresponding author, (KB).
Additional information
Funding
References
- DiSipio T, Rye S, Newman B, et al. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and Meta-analysis. The Lancet Oncol. 2013;14(6):500–515.
- Johansson K, Branje E. Arm lymphoedema in a cohort of breast cancer survivors 10 years after diagnosis. Acta Oncol. 2010;49(2):166–173.
- Casley-Smith J. Alterations of untreated lymphedema and it’s grades over time. Lymphology. 1995;28(4):174–185.
- Lymphoedema Framework. Best practice for the management of lymphoedema. International Consensus. London: MEP Ltd. 2006.
- Soran A, Ozmen T, McGuire KP, et al. The importance of detection of subclinical lymphedema for the prevention of breast cancer-related clinical lymphedema after axillary lymph node dissection; a prospective observational study. Lymphat Res Biol. 2014;12(4):289–294.
- Whitworth PW, Shah C, Vicini F, et al. Preventing breast cancer-related lymphedema in high-risk patients: the impact of a structured surveillance protocol using bioimpedance spectroscopy. Front Oncol. 2018;8:197.
- Kilgore LJ, Korentager SS, Hangge AN, et al. Reducing breast Cancer-Related lymphedema (BCRL) through prospective surveillance monitoring using bioimpedance spectroscopy (BIS) and patient directed self-interventions. Ann Surg Oncol. 2018;25(10):2948–2952.
- Yang EJ, Ahn S, Kim EK, et al. Use of a prospective surveillance model to prevent breast cancer treatment-related lymphedema: a single-center experience. Breast Cancer Res Treat. 2016;160(2):269–276.
- Ramos S, ÓDonnell L, Knight G. Edema volume, not timing, is the key to success in lymphedema treatment. Am J Surg. 1999;178(4):311–315.
- Bundred N, Foden P, Todd C, et al. Increases in arm volume predict lymphoedema and quality of life deficits after axillary surgery: a prospective cohort study. Br J Cancer. 2020;123(1):17–25.
- National Lymphedema Network (NLN) Position Paper. Risk reduction practices. Screening and measurement for early detection of breast cancer-related lymphedema. https://lymphnet.org/position-papers2011.
- Partsch H, Stout N, Forner-Cordero I, et al. Clinical trials needed to evaluate compression therapy in breast cancer related lymphedema (BCRL). Proposals from an expert group. Int Angiol. 2010;29(5):442–453.
- Specht MC, Miller CL, Russell TA, et al. Defining a threshold for intervention in breast cancer-related lymphedema: what level of arm volume increase predicts progression? Breast Cancer Res Treat. 2013;140(3):485–494.
- Mahamaneerat WK, Shyu C-R, Stewart BR, et al. Breast cancer treatment, BMI, post-op swelling/lymphedema. J Lymphoedema. 2008;3(2):38–44.
- Bucci LK, Brunelle CL, Bernstein MC, et al. Subclinical lymphedema after treatment for breast cancer: risk of progression and considerations for early intervention. Ann Surg Oncol. 2021;28(13):8624–8633.
- Mazor M, Smoot BJ, Mastick J, et al. Assessment of local tissue water in the arms and trunk of breast cancer survivors with and without upper extremity lymphoedema. Clin Physiol Funct Imaging. 2019;39(1):57–64.
- Stout NL, Pfalzer LA, Levy E, et al. Segmental limb volume change as a predictor of the onset of lymphedema in women with early breast cancer. Pm R. 2011;3(12):1098–1105.
- Forte AJ, Huayllani MT, Boczar D, et al. Use of bioimpedance spectroscopy for prospective surveillance and early diagnosis of breast cancer-related lymphedema. Breast Dis. 2021;40(2):85–93.
- Karlsson K, Nilsson-Wikmar L, Brogardh C, et al. Palpation of increased skin and subcutaneous thickness, tissue dielectric constant, and water displacement method for diagnosis of early mild arm lymphedema. Lymphat Res Biol. 2020;18(3):219–225.
- Lahtinen T, Seppälä J, Viren T, et al. Experimental and analytical comparisons of tissue dielectric constant (TDC) and bioimpedance spectroscopy (BIS) in assessment of early arm lymphedema in breast cancer patients after axillary surgery and radiotherapy. Lymphat Res Biol. 2015;13(3):176–185.
- Armer JM, Radina ME, Porock D, et al. Predicting breast cancer-related lymphedema using self-reported symptoms. Nurs Res. 2003;52(6):370–379.
- Johansson K, Holmström H, Nilsson I, et al. Breast cancer patient’s experiences of lymphedema. Scand J Caring Sci. 2003;17(1):35–42.
- Thomis S, Dams L, Fourneau I, et al. Correlation between clinical assessment and lymphofluoroscopy in patients with breast cancer-related lymphedema: a study of concurrent validity. Lymphat Res Biol. 2020;18(6):539–548.
- Mayrovitz HN, Weingrad DN, Lopez L. Assessing localized skin-to-fat water in arms of women with breast cancer via tissue dielectric constant measurements in pre- and post-surgery patients. Ann Surg Oncol. 2015;22(5):1483–1489.
- Johansson K, Ingvar C, Albertsson M, et al. Arm lymphedema, shoulder mobility and muscle strength after breast cancer treatment-a prospective 2 year study. Adv Physiother. 2001;3(2):55–66.
- Taylor R, Jayasinghe U, Koelmeyer L, et al. Reliability and validity of arm volume measurements for assessment of lymphedema. Phys Ther. 2006;86(2):205–214.
- Karges J, Mark B, Stikeleather S, et al. Concurrent validity of upper extremity volume estimates: comparison of calculated volume derived from girth measurements and water displacement volume. Phys Ther. 2003;83(2):134–145.
- Hidding JT, Viehoff PB, Beurskens CH, et al. Measurement properties of instruments for measuring of lymphedema: systematic review. Phys Ther. 2016;96(12):1965–1981.
- Mayrovitz HN, Davey S, Shapiro E. Suitability of single tissue dielectric constant measurements to assess local tissue water in normal and lymphedematous skin. Clin Physiol Funct Imaging. 2009;29(2):123–127.
- Mayrovitz HN, Mikulka A, Woody D. Minimum detectable changes associated with tissue dielectric constant measurements as applicable to assessing lymphedema status. Lymphat Res Biol. 2019;17(3):322–328.
- Scott J, Huskisson EC. Graphic representation of pain. Pain. 1976;2(2):175–184.
- Frändin K, Grimby G. Assessment of physical activity, fitness and performance in 76 year olds. Scan J Med Sci Sports. 1994;4(1):41–46.
- Paramanandam VS, Dylke ES, Clark GM, et al. Prophylactic use of compression sleeves reduces the incidence of arm swelling in women at high risk of breast cancer related lymphedema: a randomized controlled trial. J Clin Oncol. 2022;JCO2102567.
- Stout Gergich NL, Pfalzer LA, McGarvey C, et al. Preoperative assessment enables the early diagnosis and successful treatment of lymphedema. Cancer. 2008;112(12):2809–2819.