Abstract
Background
Data from the real-world setting on perioperative chemotherapy in high-risk, localized soft tissue sarcoma (STS) is limited. Real-world data (RWD) includes data derived from patients treated outside clinical trials and often captures long-term follow-up not recorded in clinical trials. The aim of this study was to provide population-based, real-world evidence on perioperative chemotherapy in localized STS.
Material and methods
Adult patients with localized STS in the extremities or trunk wall treated at Oslo University Hospital, Oslo, Norway from 1998 to 2017 were included in the study. Data were extracted from a prospectively maintained database, supplemented by retrospective review of medical records.
Results
The total study cohort included 806 patients, of whom 154 (19%) received perioperative chemotherapy. A regimen with anthracycline and ifosfamide was given in 141 of 154 cases (92%). During long-term follow-up two patients developed secondary malignancies, cardiac toxicity was registered in 11 patients (7%) and renal toxicity in 12 patients (8%). Seventy-one of 154 patients (46%) were treated outside of clinical trials and constituted the RWD cohort. The median age at surgery was slightly lower and there were more synovial sarcomas and fewer myxofibrosarcomas in the RWD cohort. No difference in chemotherapy dose intensity was observed. The estimated 5-year metastasis-free survival (MFS) in all patients receiving perioperative chemotherapy was 58%. In the RWD cohort 5-year MFS was 53% and in the clinical study cohort 61% (HR 1.24; 95% CI 0.77–2.00).
Conclusion
Long-term outcome after perioperative chemotherapy was comparable for patients treated in routine clinical practice to those in clinical trials. Secondary malignancy and cardiac toxicity were observed. The risk of serious late side effects should be included in the decision process on perioperative chemotherapy.
Introduction
Soft tissue sarcoma (STS) is a heterogeneous group of rare, malignant tumors of mesenchymal origin [Citation1]. The most common histological subtypes are gastrointestinal stromal tumor, liposarcoma and leiomyosarcoma [Citation2]. STSs can occur at any site while tumors in extremities or trunk walls are the most frequent. In localized disease, surgical resection with R0 margins is recommended, usually with the addition of pre- or postoperative radiotherapy [Citation2]. In patients with a high risk of recurrence, adjuvant or neoadjuvant (perioperative) chemotherapy with an anthracycline and ifosfamide can be considered [Citation2]. Several risk stratification tools have been developed to select high-risk patients. The TNM staging classification includes tumor size, anatomical site, lymph node involvement, distant metastasis and histological grade [Citation1], but its clinical utility to select patients for adjuvant chemotherapy is limited [Citation3]. To provide more individualized and accurate estimates of the outcome, several prognostic nomograms have been developed [Citation4–8].
The Scandinavian Sarcoma Group (SSG) has performed three studies on adjuvant chemotherapy, one randomized trial and two single-arm phases 2 studies [Citation9–11]. The randomized trial (SSG I), carried out from 1981 to 1986, did not demonstrate any effect of adjuvant single-agent doxorubicin on metastasis-free or overall survival [Citation11]. In the SSG XIII study, patients with high-risk, localized STS in the extremities or trunk wall received six cycles of doxorubicin and ifosfamide [Citation9]. High risk was defined as high grade and at least two of the following tumor characteristics: tumor size ≥8 cm, presence of necrosis and vascular invasion. The SSG XX study also included six cycles of doxorubicin and ifosfamide. High risk was defined as a high malignancy grade combined with the presence of vascular invasion and/or at least two of the following criteria: tumor size ≥8 cm, presence of necrosis and infiltrative tumor growth pattern [Citation10]. The estimated 5-year metastasis-free survival (MFS) in the SSG XIII study was 59% and in the SSG XX trial 70% [Citation9,Citation10].
Real-world data (RWD) includes data for patients who are not treated in clinical trials. It is an increasingly appreciated data source. RWD records the actual care patients receive in routine clinical practice. RWD also offers the opportunity to capture long-term follow-up results often not recorded in clinical trials. There is limited data on perioperative chemotherapy in STS from the real-world setting in the literature. Large cohorts using RWD in STS have been reported [Citation12,Citation13], but they have either not focused on perioperative chemotherapy or data has been extracted from national databases with non-uniform treatment protocols among the participating centers. Thus, there is a knowledge gap on the use of perioperative chemotherapy and outcome for STS patients in the real-world setting.
The aim of this study was to provide real-world evidence on perioperative chemotherapy. We report long-term outcomes and late toxicity in a large cohort of patients with localized STS from Oslo University Hospital treated over a 20-year period and compare characteristics and outcomes for patients treated in a real-world setting with those included in prospective clinical studies.
Material and methods
Patients
Patients with STS diagnosed between January 1, 1998 and December 31, 2017 were identified in the prospectively maintained sarcoma database at Oslo University Hospital (OUH). We excluded patients with distant metastases present on radiological assessment at diagnosis, patients with a primary tumor location other than extremities (from the shoulder girdle to the hand and from the pelvic girdle to the foot) or trunk wall, patients who did not undergo a complete surgical excision, patients with cutaneous tumors, and patients with the following histological subtypes: atypical lipomatous tumor (ALT), dermatofibrosarcoma protuberans, extraskeletal Ewing sarcoma, alveolar or embryonal rhabdomyosarcoma, and Kaposi’s sarcoma. Data were supplemented by retrospective review of medical records. From the Cancer Registry of Norway, we obtained the number of patients from the South-East Health Region who underwent resection of primary, non-metastatic STS in the same time period. By legal regulation, there is mandatory reporting of all new cancer cases in Norway to the registry. The study was approved by the OUH Data Protection Officer (approval no. 18/13611).
Diagnosis and follow-up
The histopathological classification was performed according to the 2020 WHO classification [Citation1]. Malignancy grade was classified according to the Fédération Nationale des Centers de Lutte Contre Le Cancer (FNCLCC) grading system [Citation14]. Detection of pathognomonic translocations or fusion genes was performed using FISH or RT-PCR and was performed when indicated (e.g., for myxoid liposarcoma and synovial sarcoma). A review of pathology reports was performed for all cases and in 146 cases the histological specimen was reviewed by a reference sarcoma pathologist (I.L.). Follow-up included chest X-ray or chest CT and physical examination of the primary tumor site every 3–4 months for 3 years, every 6 months years 4 and 5, and every 12 months from 6 to 10 years after surgery. MRI of the primary tumor site was performed if clinically indicated. For patients who received chemotherapy, the follow-up schedule included an echocardiogram or multigated acquisition scan (MUGA) and measurement of glomerular filtration rate (GFR) at the first follow-up visit, and after 1, 5 and 10 years.
Local treatment
All patients underwent primary tumor surgery at OUH or were referred to a sarcoma surgeon at OUH after primary surgery. If primary surgery was performed elsewhere, the patient was considered for second surgery at OUH if surgical margins were considered inadequate. Surgical margins were classified according to the closest margin by the residual tumor system. The margin was considered positive (R1) if there was a tumor at the inked surface on microscopy, and negative (R0) if there was no tumor at the inked surface. Radiotherapy was performed according to SSG guidelines (https://www.ssg-org.net).
Chemotherapy
From 1998 to 2007 patients were, as a general rule, either included in or treated according to the SSG XIII protocol, with six cycles of doxorubicin 50 mg/m2 and ifosfamide 5 g/m2 [9]. A 20% dose escalation of doxorubicin and ifosfamide was recommended for cycles 2–6 if no grade 4 toxicity occurred after the first cycle. Since 2007 patients have been included or treated according to the SSG XX protocol, where six cycles of doxorubicin 60 mg/m2 and ifosfamide 6 g/m2 was administered [Citation10,Citation15].
Statistical analysis
Associations between categorical variables were investigated using the Chi-Square test or Fisher’s exact test, and for continuous variables, the Mann-Whitney U test was used. Survival was estimated using the Kaplan-Meier method and compared with the log-rank test.
Hazard ratios (HRs) were calculated using the Cox proportional hazards regression model. The proportional hazards assumption was assessed using a visual examination of log-log survival curves and cumulative hazard function plots. Survival was calculated from the date of primary tumor surgery. For MFS, only distant metastasis was considered an event and was denoted if verified on biopsy or indisputable on X-ray or CT. For overall survival (OS), data were retrieved from the National Population Registry of Norway, and death of any cause was considered an event. Patients without an event were censored at the date of the last radiological examination for MFS and on December 31, 2021 for OS. Median follow-up was calculated using the inverse Kaplan-Meier method. IBM SPSS Statistics for Windows version 26.0 (Armonk, NY, USA) was used.
Results
Patient cohort
From 1998 to 2017, 806 patients underwent complete surgical excision of a localized STS of the extremity or trunk wall. Their clinical and histopathological characteristics are summarized in Supplementary Table 1. To investigate whether the cohort could be considered population-based, we compared our data with the Cancer Registry of Norway. From the South-East Health Region, 541 patients undergoing surgery for STS of the upper or lower extremity were reported to the Cancer Registry from 1998 to 2017. Tumors located in the trunk wall could not be reliably separated from tumors in other locations in the same anatomical regions (e.g., thorax, abdomen and retroperitoneum) and were thus not included in the data extraction from the registry. The study cohort included 498 patients with tumors in the extremities from the South-East Health Region in the same time period, corresponding to 92.1% of the number of patients reported to the Cancer Registry of Norway.
Perioperative chemotherapy cohort
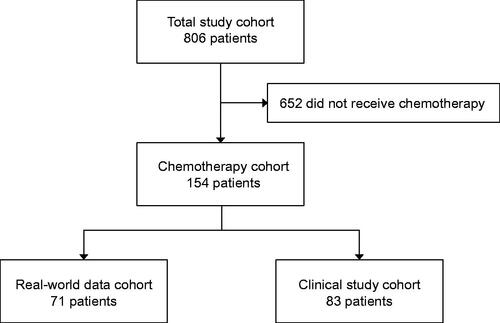
One-hundred fifty-four patients (19%) received perioperative chemotherapy, and their characteristics are summarized in . The majority of the tumors were located in the lower extremities (63%). Undifferentiated pleomorphic sarcoma was the most frequent histological subtype (27%), followed by myxofibrosarcoma (20%) and synovial sarcoma (13%). Seventy-four tumors (48%) were FNCLCC grade 3, 41 (27%) were grade 2, and in 38 cases (25%) grade was not determined. Of the latter, 30 cases were high grade using Broder’s classification (11 grade 3 and 19 grade 4) and grade could not be determined in 8 cases due to preoperative treatment. Eighty-three of the 154 patients (54%) who received chemotherapy were included in a clinical study, either the phase 2 study SSG XIII [Citation9] or the phase 2 study SSG XX [Citation10]. Seventy-one patients (46%) received systemic treatment outside of a clinical study and constituted the RWD cohort. A flowchart of the study cohort is presented in . A comparison of the demographic, clinical and histopathological characteristics of the RWD cohort and the clinical study cohort are presented in . The median age at surgery was 50 years in the RWD cohort and 54 years in the clinical study cohort (p = 0.015). There were more synovial sarcomas in the RWD cohort (20% vs 7%) and more myxofibrosarcomas in the clinical study cohort (29% vs 10%; p = 0.002).
Table 1. Demographic, clinical and histopathological characteristics of patients who received perioperative chemotherapy.
Table 2. A comparison of the demographic, clinical and histopathological characteristics of the RWD cohort and the clinical study cohort.
Chemotherapy
Of the 154 patients who received perioperative chemotherapy, 132 received postoperative treatment, 14 preoperative and 8 both pre- and postoperative therapy. The median number of cycles was 6 (range 1–10). A regimen with an anthracycline and ifosfamide was given to 141 patients (92%). Six patients were treated with etoposide, doxorubicin and ifosfamide, four patients with extraskeletal osteosarcoma received regimens including doxorubicin, ifosfamide and cisplatin, two patients with pleomorphic rhabdomyosarcoma were treated according to the ISG/SSG III regimen [Citation16], and one patient received etoposide and ifosfamide.
Chemotherapy dose intensity
The median cumulative dose of doxorubicin was 360 (range 50–480) mg/m2 in the RWD cohort and 360 (range 120–395) mg/m2 in the clinical study cohort (p = 0.719; Mann-Whitney U test). The median cumulative dose of ifosfamide was 36 (range 5–54) g/m2 in the RWD cohort and 36 (range 12–40) g/m2 in the clinical study cohort (p = 0.443; Mann-Whitney U test). Of the 141 patients who received doxorubicin and ifosfamide, 123 got 6 cycles (range 1–9). For analysis of mean cycle length per patient, we excluded patients with both pre- and postoperative treatment (n = 8). The median cycle length was 21 days (range 17–47) in the RWD cohort and 21 days (range 17–60) in the clinical study cohort (p = 0.949; Mann-Whitney U test).
Local treatment
All 154 patients who received chemotherapy underwent complete surgical resection, and 141 (92%) had a microscopically negative margin (R0; ). Ten patients (6%) received preoperative radiotherapy and 100 patients (65%) had postoperative radiotherapy. The fractionation schedule was either 1.8 Gy twice daily to 36 Gy/45 Gy (87 patients; 79%) or 1.8–2.0 Gy once daily to 50 Gy/60 Gy (27 patients; 21%). The total dose administered depended on margin status. Seven patients (5%) received regional hyperthermia and isolated limb perfusion was performed in nine cases (6%). Fifty-one patients (72%) in the RWD cohort and 59 patients (71%) in the clinical study cohort received radiotherapy (p = 1.000). Further details on local treatment and local control in the total study cohort will be reported separately.
Chemotherapy-related late toxicity
One patient developed secondary acute myeloid leukemia, and one patient was diagnosed with atypical chronic myeloid leukemia, both 7 years after chemotherapy, and both died of the disease. No other chemotherapy-related secondary malignancies were recorded. Cardiac toxicity was registered in 11 patients (7%). Nine patients had heart failure and/or reduced ejection fraction, one patient was diagnosed with sick sinus syndrome and one patient experienced aortic stenosis. Renal toxicity was recorded in 12 patients (8%), and all had reduced glomerular filtration rate without clinical symptoms of renal failure.
Outcome
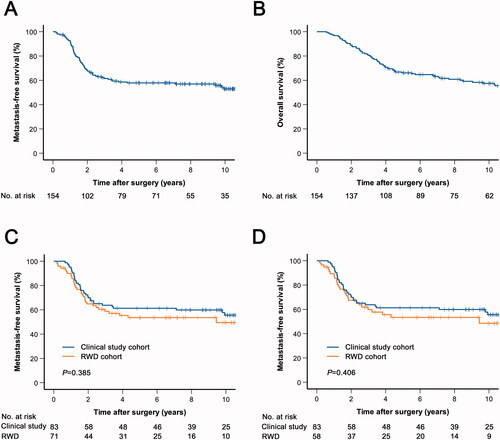
The median follow-up for metastasis-free survival (MFS) was 116 months (range 7–259). Distant metastasis was diagnosed in 67 patients (44%). Of these, 55 patients (82%) had lung metastases. The estimated five-year MFS was 58% and the 10-year MFS was 53% (). The median follow-up for overall survival (OS) was 155 months (range 48–280). Seventy-two patients (47%) died during follow-up, of whom 57 (79%) had been diagnosed with distant metastasis. Five-year OS was 66% and 10-year OS was 57% (). Five-year MFS in the RWD cohort was 53% and in the clinical study cohort 61% (HR 1.24; 95% CI 0.77–2.00; ). When only patients who received doxorubicin and ifosfamide were included in the RWD cohort (n = 58), the 5-year MFS was 53% (HR 1.24; 95% CI 0.75–2.06; ). To adjust for the potential bias introduced by the long study period, we performed a multivariable analysis including sex, age at surgery and year of diagnosis. The hazard ratio for MFS in the RWD cohort compared to the clinical study cohort was 1.26 (95% CI 0.75–2.14).
Figure 2. Kaplan-Meier curves of metastasis-free (A, C, D) and overall survival (B) in patients who received perioperative chemotherapy. (A, B) The total chemotherapy cohort. (C) The total chemotherapy cohort was stratified based on whether patients were included in a clinical trial. (D) Patients who received perioperative chemotherapy with doxorubicin and ifosfamide were stratified based on clinical trial inclusion. RWD; real-world data.
Discussion
In the present study, we report real-world evidence on perioperative chemotherapy in a large cohort of patients with localized STS of the extremities and trunk wall from Oslo University Hospital treated over a 20-year period. Patients treated in routine clinical practice had comparable baseline characteristics as patients treated in prospective clinical trials. There was no difference in chemotherapy dose intensity and patient outcome was similar between the two groups.
Several clinical studies on adjuvant chemotherapy in STS have been conducted [Citation3,Citation9,Citation10,Citation17,Citation18]. There are substantial differences in reported outcomes between studies, likely due to different inclusion criteria. Patients at high risk of metastasis and death seem to benefit from perioperative chemotherapy [Citation19], and according to current European guidelines, chemotherapy can be considered for high-risk patients [Citation2]. The SSG has used tumor size, vascular invasion, necrosis, histological grade and tumor growth pattern as criteria to define the high-risk group [Citation20,Citation21]. With these criteria, 5-year MFS in our cohort was 58%. The survival in the RWD cohort was numerically inferior to the clinical study cohort, but the difference was small and not statistically significant. The outcome for patients in clinical trials is often superior to those treated in clinical routine, since patients with advanced age, greater comorbidity and lower socioeconomic background are underrepresented in clinical trials [Citation22,Citation23]. This may limit the generalizability of trial results to the general population, in particular with toxic therapies that younger patients with less comorbidity are expected to tolerate better. In our practice, the RWD cohort was in fact younger. The reason for not including a patient in a clinical trial was not systematically recorded in the medical records in all cases and the reason for the age difference is unknown. The chemotherapy dose intensity was similar in both groups, both evaluated by the total dose administered, cycle length and delays. The vast majority of patients received the prescribed dose without major delays. Based on these observations we conclude that the SSG XIII and SSG XX trials seem to have good external validity and that the treatment given has similar effects in the general population that our center serves.
Late distant recurrences were rare. Among the 67 patients who experienced metastasis, only nine (13%) were diagnosed >3 years after surgery, and 73% were diagnosed within the first two years. Median follow-up for MFS was almost 10 years, and included imaging (at least a chest X-ray) at each follow-up visit. Our results clearly demonstrate that late distant recurrences are infrequent in this patient group and support that follow-up schedules should be less intensive after three years.
Two patients developed secondary leukemia and both died of the disease. One of the patients received postoperative radiotherapy, which also could have been a contributing factor. Cardiac toxicity was registered in 7% and even though the causal relationship to chemotherapy in these cases is unproven, it seems probable that anthracycline exposure is a contributing factor for the majority. None of the patients received radiotherapy involving the heart. Renal toxicity was recorded in 8% but was not clinically significant in any of the patients. Serious late toxicities are usually not recorded in clinical trials on adjuvant chemotherapy because it requires long-term follow-up. Nevertheless, it needs to be considered in the therapeutic decision-making process and should be discussed with the patients. If the anticipated short-term benefit of adjuvant treatment is marginal, the risk of late secondary malignancies and other late toxicities might outweigh the benefit. It remains difficult, however, how the risk of late toxicity should be weighed in the therapeutic decisions.
Our study has several strengths. All patients were treated at one sarcoma reference center with a uniform treatmentpractice. The patients were also followed at OUH after therapy, resulting in a cohort with high-quality long-term follow-up data. Our hospital is the only sarcoma center in the South-Eastern Health Region in Norway with a population of approximately 3 million people. The vast majority of patients reported to the Cancer Registry of Norway were included and the cohort is thus population-based. All histological specimens were evaluated by pathologists specialized in sarcoma diagnostics, a review of all pathology reports was performed and a significant number of specimens were reviewed by a reference pathologist. Despite these efforts, for certain histopathological variables, there are some missing data, such as for histological grade, and the results must be interpreted in light of this. Even though the OUH sarcoma database is prospectively maintained, some data were retrospectively collected. Certain subgroup analyses could not be performed due to the limited number of cases in each group, such as outcomes stratified by histological subtype. The study covers a long time period, and differences in treatment over time may exist. The two cohorts were, however, well balanced between the first and second half of the study period and multivariable analysis adjusting for the time of diagnosis gave similar results as in the unadjusted analysis. Finally, we were not able to evaluate the benefit of perioperative chemotherapy due to the lack of a comparator group.
In conclusion, we have shown in this large cohort of patients with localized STS of the extremities and trunk wall that the outcome after perioperative chemotherapy was comparable for patients treated in routine clinical practice and in clinical trials and that treatment intensity was similar in both groups. Secondary malignancy and cardiac toxicity were observed, and the risk of serious late side effects should be discussed with the patients. These data can inform the decision-making process in our daily practice. However, despite adequate local therapy and perioperative chemotherapy, almost half of the patients experience distant disease relapse, highlighting the urgent need for new and improved systemic treatment strategies for patients with high-risk localized STS.
Supplemental Material
Download MS Word (17.4 KB)Acknowledgments
We thank Malin Klötz, Stine Næss and Ane Haugen for collection and registration of data. The study has used data from the Cancer Registry of Norway. The interpretation and reporting of these data are the sole responsibility of the authors, and no endorsement by the Cancer Registry of Norway is intended nor should be inferred.
Disclosure statement
This work was supported in part by Eli Lilly & Company. The authors report no other conflicts of interest.
Data availability statement
The data that support the findings of this study are available from the corresponding author (KB) upon reasonable request.
Additional information
Funding
References
- WHO classification of tumours of soft tissue and bone tumours. 5th ed: IARC. 2020.
- Gronchi A, Miah AB, Dei Tos AP, et al. Soft tissue and visceral sarcomas: ESMO-EURACAN-GENTURIS clinical practice guidelines for diagnosis, treatment and follow-up⋆. Ann Oncol. 2021;32(11):1348–1365.
- Gamboa AC, Gronchi A, Cardona K. Soft-tissue sarcoma in adults: an update on the current state of histiotype-specific management in an era of personalized medicine. CA Cancer J Clin. 2020;70(3):200–229.
- Callegaro D, Miceli R, Bonvalot S, et al. Development and external validation of two nomograms to predict overall survival and occurrence of distant metastases in adults after surgical resection of localised soft-tissue sarcomas of the extremities: a retrospective analysis. Lancet Oncol. 2016;17(5):671–680.
- Kattan MW, Leung DH, Brennan MF. Postoperative nomogram for 12-year sarcoma-specific death. JCO. 2002;20(3):791–796.
- Mariani L, Miceli R, Kattan MW, et al. Validation and adaptation of a nomogram for predicting the survival of patients with extremity soft tissue sarcoma using a three-grade system. Cancer. 2005;103(2):402–408.
- van Praag VM, Rueten-Budde AJ, Jeys LM, et al. A prediction model for treatment decisions in high-grade extremity soft-tissue sarcomas: Personalised Sarcoma Care (PERSARC). Eur J Cancer. 2017;83:313–323.
- Acem I, van Houdt WJ, Grunhagen DJ, et al. The role of perioperative chemotherapy in primary high-grade extremity soft tissue sarcoma: a risk-stratified analysis using PERSARC. Eur J Cancer. 2022;165:71–80.
- Jebsen NL, Bruland OS, Eriksson M, et al. Five-year results from a Scandinavian Sarcoma Group Study (SSG XIII) of adjuvant chemotherapy combined with accelerated radiotherapy in high-risk soft tissue sarcoma of extremities and trunk wall. Int J Radiat Oncol Biol Phys. 2011;81(5):1359–1366.
- Sundby Hall K, Bruland OS, Bjerkehagen B, et al. Adjuvant chemotherapy and postoperative radiotherapy in high-risk soft tissue sarcoma patients defined by biological risk factors-A Scandinavian Sarcoma Group Study (SSG XX. Eur J Cancer. 2018;99:78–85.
- Alvegard TA, Sigurdsson H, Mouridsen H, et al. Adjuvant chemotherapy with doxorubicin in high-grade soft tissue sarcoma: a randomized trial of the Scandinavian Sarcoma Group. J Clin Oncol. 1989;7(10):1504–1513.
- Mahmoud O, Tunceroglu A, Chokshi R, et al. Overall survival advantage of chemotherapy and radiotherapy in the perioperative management of large extremity and trunk soft tissue sarcoma; a large database analysis. Radiother Oncol. 2017;124(2):277–284.
- Penel N, Coindre JM, Giraud A, et al. Presentation and outcome of frequent and rare sarcoma histologic subtypes: a study of 10,262 patients with localized visceral/soft tissue sarcoma managed in reference centers. Cancer. 2018;124(6):1179–1187.
- Trojani M, Contesso G, Coindre JM, et al. Soft-tissue sarcomas of adults; study of pathological prognostic variables and definition of a histopathological grading system. Int J Cancer. 1984;33(1):37–42.
- Hall KS, Bruland OS, Bjerkehagen B, et al. Preoperative accelerated radiotherapy combined with chemotherapy in a defined cohort of patients with high risk soft tissue sarcoma: a Scandinavian Sarcoma Group Study. Clin Sarcoma Res. 2020;10(1):22.
- Ferrari S, Sundby Hall K, Luksch R, et al. Nonmetastatic Ewing family tumors: high-dose chemotherapy with stem cell rescue in poor responder patients. Results of the Italian Sarcoma Group/Scandinavian Sarcoma Group III protocol. Ann Oncol. 2011;22(5):1221–1227.
- Gronchi A, Palmerini E, Quagliuolo V, et al. Neoadjuvant chemotherapy in high-risk soft tissue sarcomas: final results of a randomized trial from Italian (ISG), Spanish (GEIS), French (FSG), and polish (PSG) sarcoma groups. J Clin Oncol. 2020;38(19):2178–2186.
- Woll PJ, Reichardt P, Le Cesne A, et al. Adjuvant chemotherapy with doxorubicin, ifosfamide, and lenograstim for resected soft-tissue sarcoma (EORTC 62931): a multicentre randomised controlled trial. Lancet Oncol. 2012;13(10):1045–1054.
- Pasquali S, Pizzamiglio S, Touati N, et al. The impact of chemotherapy on survival of patients with extremity and trunk wall soft tissue sarcoma: revisiting the results of the EORTC-STBSG 62931 randomised trial. Eur J Cancer. 2019;109:51–60.
- Engellau J, Bendahl PO, Persson A, et al. Improved prognostication in soft tissue sarcoma: independent information from vascular invasion, necrosis, growth pattern, and immunostaining using whole-tumor sections and tissue microarrays. Hum Pathol. 2005;36(9):994–1002.
- Engellau J, Samuelsson V, Anderson H, et al. Identification of low-risk tumours in histological high-grade soft tissue sarcomas. Eur J Cancer. 2007;43(13):1927–1934.
- Booth CM, Tannock IF. Randomised controlled trials and population-based observational research: partners in the evolution of medical evidence. Br J Cancer. 2014;110(3):551–555.
- Meyer RM. Generalizing the results of cancer clinical trials. J Clin Oncol. 2010;28(2):187–189.