Abstract
Background
Symptoms and treatment of benign prostatic hyperplasia (BPH) or erectile dysfunction (ED) may lead to prostate cancer workup, and patterns of prescriptions before diagnosis may affect findings of pharmacoepidemiological studies. Usage of BPH and ED drugs after diagnosis may be related to prostate cancer treatment. We investigated differences in prescription rates of BPH and ED drugs among prostate cancer patients and cancer-free comparisons and between patients with localized and non-localized disease.
Material and methods
A nationwide register-based study, including all Danish men aged 50–85 years diagnosed with prostate cancer during 1998–2015 and an age-matched comparison cohort without cancer. We calculated rates of new and total prescriptions in 1-month intervals from 3 years before to 3 years after cancer diagnosis for drugs used to treat BPH and ED, overall and stratified by clinical stage.
Results
We identified 54,286 men with prostate cancer and a comparison cohort of 249,645 age-matched men. The new prescription rate for BPH drugs increased for men with prostate cancer in the year before diagnosis and peaked 1 month before diagnosis with an 18-fold higher rate. Men with prostate cancer had a higher total prescription rate of BPH drugs 3 years before diagnosis, notably among men with localized disease. Before diagnosis, the new prescription rates for ED drugs were similar among men with prostate cancer and comparisons. After diagnosis, men with prostate cancer had a 7-fold higher rate of new prescriptions for ED drugs. Among men with localized disease, the total prescription rate of ED drugs increased in the months following diagnosis.
Conclusion
Differences in prescription rates suggest increased prostate cancer surveillance among men receiving BPH drugs, whereas the post-diagnostic increase in ED drugs among men with localized disease is compatible with the increased risk of ED following prostate cancer treatment.
Background
Lower urinary tract symptoms (LUTS) and erectile dysfunction (ED) are frequent among elderly men [Citation1,Citation2]. Up to 70% of men above 60 years have LUTS and 25% have LUTS that affect the quality of life [Citation1]. ED is reported in up to 15% of men above 70 years [Citation1]. Prostate cancer is a third highly prevalent disease among elderly men, and a potential causal relationship between LUTS, ED, and prostate cancer has long been debated [Citation3,Citation4]. LUTS is considered a consequence of benign prostatic hyperplasia (BPH) whereas ED has many established underlying causes [Citation1,Citation5].
BPH is the most common cause of voiding problems, but prostate cancer may also affect the ability to void and the presences of BPH and prostate cancer are often concomitant [Citation6,Citation7]. Since BPH and prostate cancer derives from the same organ, a possible role of BPH as a precursor to prostate cancer has been discussed [Citation8]. Results from previous studies have, however, been inconsistent. Armenian et al. reported an increased risk of prostate cancer and death in men previously hospitalized with BPH, and Hammersten et al. found that fast-growing BPH could be a precursor for prostate cancer [Citation9,Citation10]. A Swedish population-based study demonstrated a slightly increased risk of prostate cancer and death in men previously hospitalized with a BPH diagnosis [Citation11]. More recently, a Danish population-based study demonstrated a higher risk of prostate cancer and death in men following a BPH diagnosis, and interestingly, the hazard ratio for prostate cancer increased with calendar time, while the hazard ratio for prostate cancer mortality remained nearly constant [Citation6]. In contrast to the Swedish study, the increased risk of subsequent prostate cancer was found regardless of initial treatment for BPH in the Danish study. Another recent Danish study showed that men with acute urinary retention had an excess risk of prostate cancer within the first 3 months after retention but no excess risk after 1 year [Citation12]. This clearly illustrates the risk of surveillance bias in prostate cancer diagnosis among men with urological symptoms since urinary retention is not considered a risk factor for prostate cancer.
In men with prostate cancer, ED has been associated with advanced disease, however, the clinically most important feature of ED is the association with prostate cancer treatment [Citation13–15]. A Swedish study of men with localized prostate cancer, 87% reported adverse symptoms related to ED and sexual inactivity after a median of 12 years after diagnosis [Citation16]. It is, however, difficult to evaluate the true prevalence of ED, since many men may have experienced erectile problems before cancer diagnosis or may not wish any help to reestablish erectile function. In a Danish study of men treated with radical prostatectomy, 22% of men with postoperative ED expressed no interest in penile rehabilitation [Citation15].
Previous pharmacoepidemiological studies have investigated associations between drugs used to treat both BPH and ED and prostate cancer [Citation17–19]. However, seeking medical advice or being treated for either voiding problems or ED may be the first steps leading to prostate cancer workup, and patterns of prescriptions before diagnosis could therefore affect findings of studies investigating the association between BPH and ED drugs and prostate cancer risk. The use of these drugs after diagnosis may also be related to treatment of prostate cancer. We aimed to examine the use of BPH and ED drugs before prostate cancer diagnosis to elucidate potential surveillance biases or pre-cursor effects and thereby inform planning and interpretation of future pharmacoepidemiological studies investigating associations between drugs for BPH, ED, and prostate cancer risk, and to examine prescription patterns after diagnosis to detect associations between BPH and ED drugs and prostate cancer treatment.
Material and methods
Based on information from Danish nationwide demographic and health registries, we investigated pre- and post-diagnostic prescription rates of drugs used to treat BPH and ED among men with a prostate cancer diagnosis and a comparison cohort.
Data sources
We retrieved information from the Danish Civil Registration System administering the unique personal identification number, which enables unambiguous linkage at an individual level between Danish registries, and also contains continuously updated information on vital status, and migration [Citation20]. The Danish Cancer Registry holds almost complete and accurate records of incident cancer cases in Denmark since 1943 and includes information on clinical stage recorded as ‘localized’, ‘regional’, ‘distant’, or ‘unknown’ until 2003 and according to the tumor node metastasis (TNM) system from 2004 onwards [Citation21]. The Danish National Prescription Registry holds information on all drug prescriptions dispensed at Danish pharmacies since 1 January 1995 [Citation22]. All drugs are categorized according to the Anatomical Therapeutic Chemical (ATC) Classification System [Citation23]. The Danish National Patient Register holds information on all hospital admissions and procedures in Denmark, with records of non-psychiatric hospital admissions since 1977 and on outpatient and psychiatric contacts since 1995 [Citation24].
Study population
We identified all men between 50 and 85 years with a prostate cancer diagnosis (International Classification of Diseases, 10th Revision: C61.9) recorded in the Cancer Registry from 1 January 1998 to 31 December 2015. Men with previous cancer (except non-melanoma skin cancer) were excluded. We categorized prostate cancer patients according to the clinical stage at the time of diagnosis as localized, non-localized, or unknown (see for stage algorithm). For the comparison cohort, we randomly selected up to five male population controls for each prostate cancer patient, matched on date of birth and with no history of cancer at the time of diagnosis (index date) of the corresponding prostate cancer patient.
Study drugs
We obtained information from the Prescription Registry on filled prescriptions for drugs used to treat BPH (ATC code G04C) and ED (ATC code G04BE) (see ) from 3 years before to 3 years after the index date. We investigated rates of both new and total prescriptions. A new prescription was defined as a prescription within the study drug class, i.e. BPH or ED drugs, filled by a man with no previous prescriptions within the same drug class for the preceding 2 years. Total prescriptions were defined as any prescription filled during the study period within the respective drug classes.
Statistical analyses
All men were followed from 3 years before to 3 years after the index date, or to the date of death or migration, whichever came first. We conducted descriptive analyses of characteristics at index date for men with prostate cancer and the comparison cohort and for prostate cancer patients stratified by stage at the time of diagnosis. Based on information from the Danish National Patient Register, we estimated the Charlson’s Comorbidity Index to compare comorbidity between prostate cancer patients and the comparison cohort [Citation24,Citation25]. We estimated prescription rates for each drug class in 1-month intervals during 3 years before to 3 years after the index date by counting the number of prescriptions (new or total) and dividing it by the number of person-months in that interval. The prescription rates before and after index date were reported as prescription rates per 100 person-months with 95% confidence intervals (CI) for all men diagnosed with prostate cancer, overall and stratified by localized and non-localized disease, compared with the corresponding matched comparisons.
Results
Characteristics of the study population
The study population comprised 54,286 men with prostate cancer and a comparison cohort of 249,645 age-matched men. We identified 30,712 prostate cancer patients with localized disease and 12,884 with non-localized disease at the time of diagnosis. Due to missing information on stage, 10,690 (20%) of the patients were excluded from the stratified analyses. With a median age of 68.8 years (interquartile range [IQR], 63.7–74.1) at the time of diagnosis, men diagnosed with localized disease tended to be younger than men with non-localized disease (median age of 72.4 years; IQR, 66.2–78.1) (). Men diagnosed with non-localized disease were more likely to have comorbidities compared with men diagnosed with localized disease.
Table 1. Descriptive characteristics at diagnosis (index date) of the comparison cohort and prostate cancer patients diagnosed from 1998 to 2015, overall and stratified by clinical stage at the time of diagnosis.
Drugs used for benign prostatic hyperplasia
The rate of new prescriptions for BPH drugs was similar for men with prostate cancer and men in the comparison cohort until approximately 1 year before diagnosis (). Hereafter, the rate increased slightly among men with subsequent prostate cancer up to 6 months before diagnosis, followed by a rapid increase with the highest rate observed in the month before diagnosis (3.17 new prescriptions per 100 person-months; 95% CI, 3.02–3.32) corresponding to a more than 18-fold higher rate than among men in the comparison cohort (0.17 new prescriptions per 100 person-months; 95% CI, 0.15–0.18). Following diagnosis, the rate of new prescriptions declined rapidly and leveled out at a similar rate as among men in the comparison cohort. In analyses stratified by clinical stage, the rates of new prescriptions of BPH drugs were similar for men with localized and non-localized disease.
Figure 1. Rates of new and total prescriptions of drugs for benign prostatic hyperplasia among 54,286 Danish prostate cancer patients diagnosed from 1998 to 2015 and a comparison cohort of 248,645 age-matched men, overall and stratified by clinical stage at the time of diagnosis. Note the difference in the Y-axis scales between new and total prescriptions.
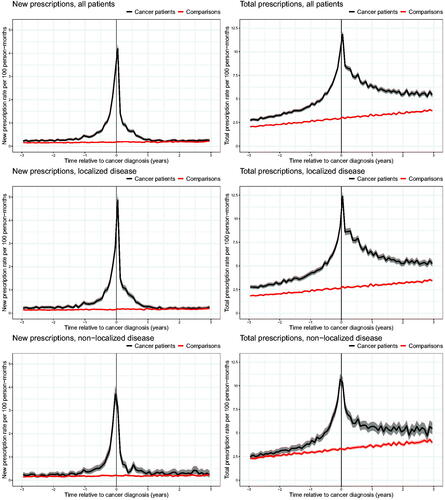
Throughout the 3 years before diagnosis, the rate of total prescriptions for BPH drugs was higher among men subsequently diagnosed with prostate cancer than among men in the comparison cohort (). Three years before diagnosis, men with prostate cancer had a rate of 2.75 prescriptions per 100 person-months (95% CI, 2.61–2.89) compared to 2.06 prescriptions per 100 person-months (95% CI, 2.00–2.12) among men in the comparison cohort. Among men with prostate cancer, the total prescription rate increased from 3 years before diagnosis and peaked around the time of diagnosis with a rate of 11.84 prescriptions per 100 person-months (95% CI, 11.55–12.13). Most of this difference was seen among men subsequently diagnosed with localized prostate cancer since the rate among men diagnosed with non-localized disease did not deviate markedly from that of the men in the comparison cohort until 1 year before diagnosis. In the comparison cohort, the total prescription rate increased steadily throughout the study period. Shortly after diagnosis, the rate of total prescriptions for drugs used to treat BPH declined among prostate cancer patients but remained higher than among men in the comparison cohort throughout the study period.
Drugs used for erectile dysfunction
The rates of new prescriptions for ED drugs were similar among men diagnosed with prostate cancer and the comparison cohort until shortly before diagnosis (). One month before diagnosis, the rate of new prescriptions increased three-fold among men subsequently diagnosed with prostate cancer compared with the comparison cohort, i.e. rates of 0.47 new prescriptions per 100 person-months (95% CI, 0.41–0.53) among prostate cancer patients and 0.14 (95% CI, 0.13–0.16) among men in the comparison cohort. After diagnosis, the rate of new prescriptions increased substantially among men with prostate cancer (1.37 new prescriptions per 100 person-months; 95% CI, 1.27–1.47), leading to a more than seven-fold higher rate 6 months after diagnosis compared with the comparison cohort. From this point, the rate of new prescriptions gradually decreased among men with prostate cancer, reaching a similar rate as in the comparison cohort 3 years after diagnosis.
Figure 2. Rates of new and total prescriptions of drugs for erectile dysfunction among 54,286 Danish prostate cancer patients diagnosed from 1998 to 2015 and a comparison cohort of 248,645 age-matched men, overall and stratified by clinical stage at the time of diagnosis. Note the difference in the Y-axis scales between new and total prescriptions.
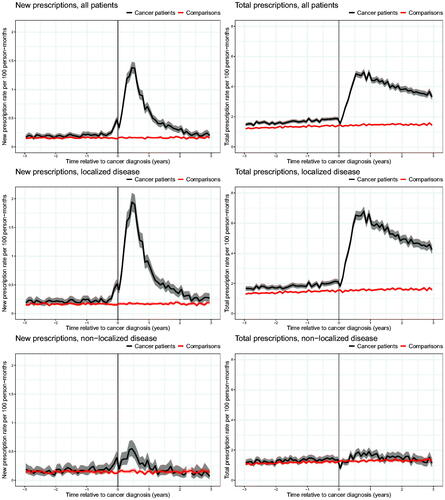
The rate of total prescriptions for ED drugs was slightly higher among men subsequently diagnosed with prostate cancer than among men in the comparison cohort within 3 years before diagnosis (). Three years before diagnosis, men with subsequent prostate cancer had a rate of 1.48 prescriptions per 100 person-months (95% CI, 1.38–1.58) compared with 1.20 prescriptions per 100 person-months (95% CI, 1.15–1.24) among men in the comparison cohort. In the first year after diagnosis, the total prescription rate among men with prostate cancer increased substantially and remained elevated throughout the study period. Three years after diagnosis, men with prostate cancer had a rate of 3.33 prescriptions per 100 person-months (95% CI, 3.14–3.53) compared with 1.44 prescriptions per 100 person-months (95% CI, 1.38–1.49) among men in the comparison cohort.
The analyses stratified by stage revealed a major difference in the total prescription rate between patients with localized and non-localized disease after diagnosis. Among men with localized disease, the rate increased dramatically in the months following diagnosis, whereas no material changes were observed among men with non-localized disease.
Discussion
Our study illustrates that a prostate cancer diagnosis has a considerable influence on the patterns of prescriptions for drugs used to treat BPH and ED. The patterns, however, differed significantly between the two drug classes, before and after diagnosis, and between men diagnosed with localized and non-localized disease.
The increasing rate of new prescriptions for BPH drugs among men with prostate cancer within the year before diagnosis may indicate that men initiating treatment for BPH are also likely to undergo diagnostic workup for prostate cancer. This may be due to either extended surveillance or that the voiding problems are a consequence of both cancer and BPH. We anticipated that men with voiding problems experience a close surveillance in the health care system, which most likely would increase their risk of being diagnosed with a localized prostate cancer but our finding of similar patterns in the new prescription rates among men with localized and non-localized disease indicate that other factors than extended surveillance influence the risk of subsequently being diagnosed with prostate cancer. However, men subsequently diagnosed with prostate cancer also had a higher total prescription rate at least 3 years before diagnosis, which was most pronounced among men diagnosed with localized disease, supporting that extended surveillance before diagnosis more than a causal effect explain the association between BPH and prostate cancer as previously shown in a Danish setting [Citation6].
As expected, the total rates of prescriptions of BPH drugs increased among men in the comparison cohort throughout the study period, indicating an increased prevalence of BPH with increasing age [Citation2]. This increase also explains parts of the increased rate among men with prostate cancer, however, the pre-diagnostic rate of BPH drugs was markedly larger among the prostate cancer patients, especially up to the time of diagnosis.
Before prostate cancer diagnosis, the rate of new prescriptions for ED drugs only differed marginally between men subsequently diagnosed with prostate cancer and men in the comparison cohort, indicating that men treated for ED are not under increased surveillance for prostate cancer. This is further supported by our finding of almost identical rates of new prescriptions before diagnosis between men with localized and non-localized disease. In the first year after diagnosis, we observed a peak in new prescriptions of ED drugs, but only among men with localized disease. This finding is consistent with ED being a frequent side effect of curatively intended treatment for prostate cancer, and our finding thus reflects an increased need for drugs to manage ED among patients with localized disease of whom many have undergone such treatment [Citation16]. The total prescription rate of ED drugs also increased among men with prostate cancer in the year after diagnosis and, although declining, remained elevated throughout the study period, however, only among men with localized disease. The decline following the first year may reflect spontaneous recovery of erectile function, no interest in penile rehabilitation, or lack of effect [Citation14,Citation15,Citation26].
Based on our findings for new prescriptions, it seems sufficient to use a 1-year lag period in pharmacoepidemiological studies investigating associations between ED drugs and prostate cancer. It is, however, not possible to define an appropriate lag period for BPH drugs based on the present study, since the total prescription rates in our study were higher among prostate cancer patients than among men in the comparison cohort from the beginning of the exposure period 3 years before diagnosis.
The increased rates of total prescriptions of BPH and ED drugs among prostate cancer patients before diagnosis indicate a higher prevalence of BPH and ED several years before diagnosis. In a recent study, we found that men subsequently diagnosed with prostate cancer less frequently used some commonly used drugs including antidepressants, beta-blockers, and antidiabetics compared with the comparison cohort [Citation27]. Moreover, in the present study, we found that a lower proportion of patients had a score of two or more on the Charlson’s Comorbidity Index compared with the comparison cohort. Therefore, we do not believe that men with subsequent prostate cancer, in general, have a higher prevalence of comorbidities, and thus our results appear specific for conditions with urological symptoms.
The patterns of prescription rates of BPH drugs after diagnosis are most likely influenced by prostate cancer treatment. In Denmark, approximately a quarter of all prostate cancer patients undergo radical prostatectomy and are therefore unlikely to receive any medication for BPH [Citation28]. Many patients with non-localized disease are likely to undergo some type of endocrine manipulation, either as treatment in combination with external beam radiation [Citation29] or as endocrine therapy alone, which will reduce prostate volume and in many cases relieve voiding problems [Citation30]. This may explain parts of the observed decrease in the rates of both new and total prescriptions for BPH after diagnosis.
The strengths of our study include a large study population, covering virtually all men diagnosed with prostate cancer in Denmark in the study period, due to the high completeness of the Danish Cancer Registry [Citation21]. The possibility to stratify analyses by stage at the time of diagnosis was another major strength of our study since patients with localized and non-localized disease differ in many parameters including age, comorbidity, socioeconomic position, and prostate cancer treatment. In Denmark, all drugs used for the treatment of BPH and ED are available by prescription only, and thus, the complete and high-quality information from the Prescription Registry provided a valid measure of the use of BPH and ED drugs. We did, however, lack information on the indication of use for the included drugs, but in general, the included drugs are highly specific for BPH and ED.
In conclusion, we found differences in prescription rates of BPH and ED drugs between men with prostate cancer and the comparison cohort as well as between patients with localized and non-localized disease. Increased pre-diagnostic rates of prescriptions may be explained by extended surveillance especially among men treated for BPH and to a lesser extent for ED, however, our results cannot rule out an association between BPH and subsequent prostate cancer. The observed differences in prescription rates among patients diagnosed with localized and non-localized disease emphasize the importance of including stage as a parameter in pharmacoepidemiological studies investigating associations between drugs for the treatment of BPH and ED and prostate cancer risk and outcomes. The increase in prescriptions of drugs used to treat ED in men with localized disease is compatible with the increased risk of erectile dysfunction following prostate cancer treatment.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Access to data can be obtained by request to the corresponding author and the Danish data protection authorities.
Additional information
Funding
References
- De Nunzio C, Roehrborn CG, Andersson K-E, et al. Erectile dysfunction and lower urinary tract symptoms. Eur Urol Focus. 2017;3(4-5):352–363.
- Devlin CM, Simms MS, Maitland NJ. Benign prostatic hyperplasia – what do we know? BJU Int. 2021;127(4):389–311.
- Larønningen S, Ferlay J, Bray F, et al. NORDCAN: cancer incidence, mortality, prevalence and survival in the Nordic countries, version 9.1. Nord Cancer Incid Mortality, Preval Surviv Nord Countries, Version 91. 2021.
- Kvåle R, Myklebust T, Engholm G, et al. Prostate and breast cancer in four Nordic countries: a comparison of incidence and mortality trends across countries and age groups 1975–2013. Int J Cancer. 2017;141(11):2228–2242.
- Kim EH, Larson JA, Andriole GL. Management of benign prostatic hyperplasia. Annu Rev Med. 2016;67:137–151.
- Ørsted DD, Bojesen SE, Nielsen SF, et al. Association of clinical benign prostate hyperplasia with prostate cancer incidence and mortality revisited: a nationwide cohort study of 3,009,258 men. Eur Urol. 2011;60(4):691–698.
- Ørsted DD, Bojesen SE. The link between benign prostatic hyperplasia and prostate cancer. Nat Rev Urol. 2013;10(1):49–54.
- Bostwick DG, Burke HB, Djakiew D, et al. Human prostate cancer risk factors. Cancer 2004;101(10 Suppl):2371–2490. [cited 2021 Dec 9]
- Armenian H, Lilienfeld A, Diamond E, et al. Relation between benign prostatic hyperplasia and cancer of the prostate. Lancet. 1974;304(7873):115–117.
- Hammarsten J, Högstedt B. Calculated fast-growing benign prostatic hyperplasia. Scand J Urol Nephrol. 2002;36(5):330–338.
- Chokkalingam AP, Nyrén O, Johansson J-E, et al. Prostate carcinoma risk subsequent to diagnosis of benign prostatic hyperplasia: a population-based cohort study in Sweden. Cancer. 2003;98(8):1727–1734.
- Bengtsen MB, Farkas DK, Borre M, et al. Acute urinary retention and risk of cancer: population based Danish cohort study. BMJ 2021;375:n2305.
- Gaither TW, Awad MA, Osterberg EC, et al. The natural history of erectile dysfunction after prostatic radiotherapy: a systematic review and meta-analysis. J Sex Med 2017;14(9):1071–1078.
- Plym A, Folkvaljon Y, Garmo H, et al. Drug prescription for erectile dysfunction before and after diagnosis of localized prostate cancer. J Sex Med. 2014;11(8):2100–2108.
- Haahr MK, Azawi NH, Andersen LG, et al. A retrospective study of erectile function and use of erectile aids in prostate cancer patients after radical prostatectomy in Denmark. Sex Med. 2017;5(3):e156–e162.
- Carlsson S, Drevin L, Loeb S, et al. Population-based study of long-term functional outcomes after prostate cancer treatment. BJU Int. 2016;117(6B):E36–E45.
- Murtola TJ, Tammela TLJ, Määttänen L, et al. Prostate cancer incidence among finasteride and alpha-blocker users in the Finnish Prostate Cancer Screening Trial. Br J Cancer. 2009;101(5):843–848.
- Murtola TJ, Karppa EK, Taari K, et al. 5-Alpha reductase inhibitor use and prostate cancer survival in the Finnish Prostate Cancer Screening Trial. Int J Cancer. 2016;138(12):2820–2828.
- Wu Y, Wang Y, Gu Y, et al. Prostate cancer risk and prognostic influence among users of 5-alpha-reductase inhibitors and alpha-blockers: a systematic review and meta-analysis. Urology. 2020;145:216–223.
- Pedersen CB. The Danish civil registration system. Scand J Public Health. 2011;39(7 Suppl):22–25.
- Gjerstorff ML. The Danish cancer registry. Scand J Public Health. 2011;39(7 Suppl):42–45.
- Kildemoes HW, Sørensen HT, Hallas J. The Danish national prescription registry. Scand J Public Health. 2011;39(7 Suppl):38–41.
- Norwegian Institute of Public Health. WHO collaborating Centre for drug statistics methodology, guidelines for ATC classification and DDD assignment 2022. Oslo (Norway): WHO Collaborating Centre for Drug Statistics Methodology; 2021. [cited 2022 Feb 15]. Available from: http://www.whocc.no/atcddd/.
- Lynge E, Sandegaard JL, Rebolj M. The Danish national patient register. Scand J Public Health 2011;39(7 Suppl):30–33.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Fode M, Sønksen J. Sexual function in elderly men receiving Androgen Deprivation Therapy (ADT). Sex Med Rev. 2014;2(1):36–46.
- Larsen SB, Dehlendorff C, Skriver C, et al. Prescription rates for commonly used drugs before and after a prostate cancer diagnosis. Cancer Causes Control. 2022;33(3):417–428.
- Dansk Urologisk Cancer Gruppe. Årsrapport 2018 [Internet]. 2019 [cited 2021 Sep 7]. Available from: www.rkkp.dk.
- Widmark A, Klepp O, Solberg A, et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet 2009;373(9660):301–308.
- Klarskov LL, Klarskov P, Mommsen S, et al. Effect of endocrine treatment on voiding and prostate size in men with prostate cancer: a long-term prospective study. Scand J Urol Nephrol. 2012;46(1):37–43.
Appendix
Appendix Table 1. Definition of clinical stage.
Appendix Table 2. List of drugs included in the included drug classes.
