Abstract
Background
The ideal timing for the initiation of chemotherapy and radiation therapy (RT) in the use of definitive chemoradiation (CRT) for patients with head and neck cancer is not well established. We sought to evaluate the impact of the timing of initiating these two modalities on clinical outcomes.
Materials and methods
Patients with squamous cell carcinoma of the head and neck who were treated using definitive chemoradiation from 2012 to 2018 were identified. Patients undergoing re-irradiation, post-op CRT, had recurrent or second primaries, or ECOG 3-4 were excluded. Outcomes including locoregional control (LRC), distant control (DC), progression-free survival (PFS), and overall survival (OS) were estimated and compared between subgroups of the cohort based on the timing in which chemotherapy or RT were initiated: chemotherapy first, same day start, within 24 h, or start on Monday/Tuesday/Wednesday.
Results
A total of 131 patients were included for analysis consisting of oropharynx (64%), larynx (22.9%), nasopharynx (6.9%), hypopharynx (3.1%), oral cavity (1.5%), and unknown primary (1.5%). Chemotherapy was administered as bolus cisplatin every 3 weeks in 40% of patients and weekly cisplatin in 60% with a median cumulative dose of 240 mg/m2. In the multivariable analysis (MVA), starting chemotherapy before RT was associated with improved LRC (HR 0.33, 95% CI: 0.11–0.99). Three-year LRC for patients starting chemotherapy first was 90.9% compared to 78.2% in those starting RT first. In the MVA, cisplatin regimen and cumulative cisplatin dose were associated with improved OS, while no factors were significantly associated with DC or PFS.
Conclusion
Starting chemotherapy prior to radiation therapy improves LRC, but did not impact DC, PFS, or OS. Clinical outcomes were not different when stratifying by the other differences in the timing of initiating chemotherapy or RT.
Background
Head and neck cancer continues to be a common cancer despite the overall decline in rates of tobacco use in the United States with an estimated 65,000 new cases in 2019 [Citation1,Citation2]. Treatment of head and neck cancer often requires surgery, radiation therapy, and/or chemotherapy. Traditionally, surgery and radiation therapy have been the predominant modalities for curative treatment. Radiation therapy was often employed for locally advanced disease that was too extensive and would require morbid surgical resection.
In patients undergoing curative treatment, it was hopeful that the addition of chemotherapy would improve outcomes with improved response rates and survival. Multiple studies investigated the addition of chemotherapy, often in the induction or adjuvant setting, and the results showed minimal impact in terms of survival. After establishing comfort with chemotherapy used in the single modality setting, studies were developed to investigate the use of chemotherapy synchronously with radiation therapy. An initial pilot study with RTOG 81-17 investigated the concurrent use of cisplatin and radiation therapy [Citation3]. The initial and long-term results found concurrent chemoradiation to be effective and safe in advanced head and neck cancer, prompting the development of prospective randomized trials [Citation3,Citation4]. Several meta-analyses have established that concurrent use of chemotherapy provides a 5–10% reduction in mortality [Citation5–7].
The use of concurrent chemoradiation is now an established paradigm for patients with locally advanced head and neck cancer for definitive treatment. Cisplatin was initially designed to be administered at a dose of 100 mg/m2 every 3 weeks for three total doses during the course of radiotherapy. Concerns for intolerance with this regimen in patients with poor performance status or comorbidities led to the use of low-dose weekly cisplatin. Weekly cisplatin showed similar survival outcomes in a meta-analysis examining locally advanced squamous cell carcinoma of the head and neck undergoing definitive or post-op chemoradiation [Citation8].
While many of the prospective trials mandated that chemotherapy and radiation start on day one, there has not been a prospective trial examining the impact of the timing of the start of both chemotherapy and radiation therapy. The current NRG protocols examining patients receiving chemoradiation mandate that cisplatin should be given within 24 h before or after the first scheduled radiation treatment (NCT03952585). In real-world practice, there may be numerous barriers to initiation of both chemotherapy and radiation therapy on the same day. These may include but are not limited to institutional barriers (systematic, logistical, administrative) or patient-specific (sociodemographic issues, transportation, patient/family time, or work constraints). These hindrances can significantly affect an institution’s ability to open such restrictive clinical trials or a patient’s willingness/ability to enroll on such a clinical trial. Considering the growth in our understanding of methods to identify and overcome gaps in healthcare equality, this seemingly small issue may have large downstream effects on patient care and clinical trial enrollment/success.
There is a significant lack of data in the literature investigating sequencing of chemotherapy and radiotherapy start in head and neck cancer. We sought to investigate the impact of varied start times of both chemotherapy and radiation therapy at our institution in the cohort of patients undergoing definitive chemoradiation for locally advanced head and neck cancer.
Materials and methods
Patient selection and treatment
In this institutional review board approved study, patients with squamous cell carcinoma of the head and neck that were treated with definitive intent using concurrent chemotherapy and radiation between 2012 and 2018 were selected from an institutional head and neck cancer database. Study data were collected and managed using REDCap electronic data capture tools hosted by the Wake Forest Clinical and Translational Science Institute [Citation9]. A total of 323 patients were identified. Exclusions (number) were as followed: non-SCC histology (n = 17), recurrent or second primary head and neck cancer (n = 19), ECOG performance status 3–4 (n = 1), metastatic disease (n = 3), re-irradiation (n = 1), postoperative CRT (n = 81), non-cisplatin chemotherapy regimen (n = 48), inadequate CRT (weekly cisplatin <40 mg/m2 or RT dose < 60 Gy, n = 21), and unknown date of chemotherapy start (n = 1). Patient demographic, tumor, and treatment characteristics were abstracted from the electronic medical record.
Patients were managed based on the recommendations of a multidisciplinary tumor board. Work up included clinical evaluations by otolaryngology, radiation oncology, medical oncology, speech language pathology, and dietetics. Patients underwent disease staging with flexible fiberoptic nasopharyngolaryngoscopy, contrast-enhanced computed tomography (CT) imaging of the head/neck and chest, or positron emission tomography (PET/CT). Chemotherapy was delivered concurrently with radiotherapy with high-dose cisplatin preferred but with the alternate option including low-dose weekly cisplatin 40 mg/m2 at the discretion of the medical oncologist. Radiotherapy was performed according to institutional practice as previously described [Citation10]. Patients underwent CT simulation in the supine position with thermoplastic mask for immobilization. Gross tumor volume (GTV) of the primary and involved lymph nodes (identified by PET/CT or contrast-enhanced CT) were delineated and expanded by 0.5–1 cm to create a clinical target volume (CTV). Elective nodal regions at risk were also delineated as individual CTVs. The planning target volume (PTV) was created by a 0.3–0.5 cm isotropic expansion. Dose was prescribed with 70 Gy in 35 daily fractions to the gross disease/high-risk CTVs with the intermediate- and low-risk regions receiving 63 Gy and 56 Gy, respectively, using a simultaneous integrated boost technique.
Outcomes and follow-up
Patients were seen in follow up within 4–6 weeks post-treatment with routine clinical follow up every 2–3 months thereafter. Post-treatment imaging was obtained with a contrasted CT or PET/CT at 2–3 months post-treatment. Disease recurrence was defined as any radiographic or pathologic evidence of disease in the primary site (local), neck (regional), or areas outside the neck (distant). Outcome measures were calculated from the time from RT start to the event of interest or censored at last date seen in clinic. Locoregional control (LRC) was defined as freedom from local (at the primary site) or regional (in the neck) recurrence. Distant control (DC) was defined as freedom from distant metastasis. Progression-free survival (PFS) was defined as freedom from disease recurrence (local, regional, or distant), last clinical follow-up, or death, whichever occurred first. Overall survival (OS) was defined as time to death from any cause.
Statistical analysis
Summary statistics, including medians and the 25th and 75th percentile for continuous measures and frequencies and percentages for categorical data, were calculated for all study variables. Study groups were compared using independent t tests for continuous and Fisher’s exact tests for categorical variables. Survival times were calculated as the interval between the start of radiation therapy and the event of interest; if no event was observed, the patient was censored at date of last follow up. The Kaplan–Meier method was utilized to estimate survival probabilities in all time-to-event outcomes. Cox proportional hazards models assessed the relationship between survival times and independent measures; these models generated hazard ratios and 95% confidence interval. Stepwise regression was used to find a best-fit model for time-to-event outcomes. The variables entered in the univariable model included: age, gender, ECOG, Charlson Comorbidity, stage, chemotherapy started first, modalities (chemotherapy and radiation therapy) started same day, modalities (chemotherapy and radiation therapy) started within 24 h, chemoradiation started on Monday/Tuesday/Wednesday (versus Thursday/Friday), concurrent chemotherapy (bolus versus weekly). The statistical significance level was set at α = 0.05. SAS (version 9.4, Cary, NC, USA) was used for all analyses.
Results
Patient and tumor characteristics
A total of 131 patients were included in the analysis. Baseline characteristics are summarized in . The median age was 58 years and a majority were male. A majority had an ECOG of 0–1, 49.6% had a Charlson comorbidity index of 0–1, and 77% were either current or former smokers. The primary site of involvement included: oropharynx (64%), larynx (22.9%), nasopharynx (6.9%), hypopharynx (3.1%), oral cavity (1.5%), and unknown primary (1.5%). Of patients with a primary site of the oropharynx, 83.3% were HPV-positive.
Table 1. Patient baseline characteristics by concordant day of start of chemo and radiation therapy or discordant.
All patients were treated with definitive chemoradiation and treatment details are summarized in . The median dose and number of fractions delivered during the course of RT was 70 Gy and 35 fractions. RT was delivered using IMRT/VMAT in 92%, a combination of 3 D/IMRT in 7%, and 3 D alone in 1%. Chemotherapy was given as bolus cisplatin every 3 weeks in 40% of patients and weekly cisplatin in 60%. The median cumulative cisplatin dose given was 240 mg/m2. Chemotherapy and radiation therapy started on the same day in 47% of patients and radiation therapy was started prior to chemotherapy in 61% of patients (Supplemental Figure 1).
Table 2. Summary of treatment characteristics.
Impact of timing of radiotherapy and chemotherapy on locoregional control
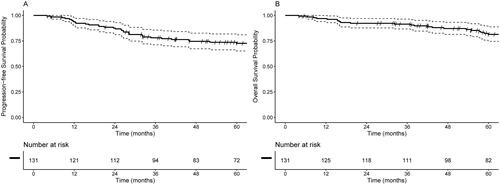
The median follow up was 2.9 years. Overall locoregional control at 3 years was 83.1% (95% CI: 76.1, 90.1). Chemotherapy prior to RT was associated with significantly improved locoregional control (HR 0.33, 95% CI: 0.11, 0.99) in the univariable model. There were no significant differences noted for LRC based on same day versus different day start, treatments starting within 24 h versus separated by >24 h, starting CRT on Monday/Tuesday/Wednesday versus Thursday/Friday. In the multivariable model, chemotherapy starting before RT was associated with significant improvement in LRC (HR 0.33, 95% CI: 0.11–0.99) (). Three-year locoregional control for patients who started chemotherapy first was 90.9% (95% CI: 82.4, 99.4) compared to 78.2% (95% CI: 68.4, 88.0) for those that started radiation therapy first (). Three-year LRC was 86.1% (95% CI: 76.5, 95.7) for patients who started chemotherapy and radiation therapy on the same day versus 80.9% (95% CI: 71.2, 90.7) for those starting on different days ().
Figure 1. Kaplan–Meier plot of locoregional control by chemotherapy first versus radiotherapy first (A) and by same day versus different day start (B).
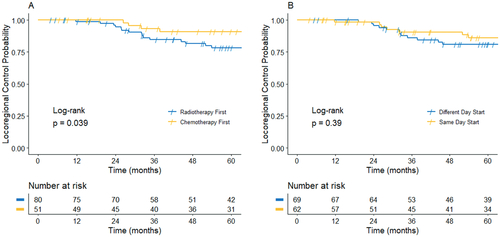
Table 3. Univariable and multivariable proportional hazards on locoregional control.
Impact of timing and other clinical factors on survival outcomes
The median time-to-first event (death or any recurrence) was not reached and 3-year event-free rate was 69.1% (95% CI: 60.6, 77.6) (). Similarly, median OS has not been attained, with a 3-year OS of 77.8% (95% CI: 70.0, 85.7) (). There were no significant differences in OS based on same day versus different day start (HR 0.65, 95% CI: 0.75, 3.17), treatments starting within 24 h versus separated by >24 h (HR 1.68, 95% CI: 0.81, 3.45), starting CRT on Monday/Tuesday/Wednesday versus Thursday/Friday (HR 0.50, 95% CI: 0.23, 1.09).
Factors significantly associated with OS in the univariable model included: ECOG performance status (HR 2.14 per 1 unit increase, 95% CI: 1.08, 4.24), stage IVB versus II/III (HR 5.88, 95% CI: 1.52, 22.7), high-dose cisplatin versus weekly cisplatin (HR 0.43, 95% CI: 0.19, 0.99), and cumulative cisplatin dose <200 (HR 3.41, 95% CI: 1.57, 7.42). In the multivariable model, cumulative cisplatin dose was significantly associated with OS. If the model is reduced in a stepwise fashion until only significant variables remain, the only remaining independent factors are cisplatin regimen (HR 0.41, 95% CI: 0.17, 0.94) and cumulative dose (HR 3.61, 95% CI: 1.65, 7.86).
In assessing the relationship between PFS and predictor variables, only ECOG (HR 2.02, 95% CI: 1.09, 3.73) was found to have prognostic significance in the univariable model; no other variables were found to be significant in the stepwise multivariable models. No independent measures were found to be significantly associated with DC; the p-value for same day versus different day start is 0.27 (HR = 0.55, 95% CI: 0.19, 1.62), while the p-value is 0.64 (HR = 0.77, 95% CI: 0.26, 2.27) for comparing chemotherapy first versus radiation therapy first.
Discussion
There are no prospective clinical studies that have specifically assessed the timing of the initiation of chemotherapy and radiation therapy in patients with head and neck cancer. In this retrospective review of a cohort of patients undergoing definitive concurrent chemotherapy and radiation therapy we analyzed disease control and survival outcomes based on stratifying the differing temporal initiation of chemotherapy or radiation. We found that patients who received chemotherapy prior to initiation of radiation therapy did have significantly improved locoregional control with a HR of 0.33 on multivariable analysis.
Many trials since the 1980s have investigated combining chemotherapy and radiation therapy. It was demonstrated for multiple sites in head and neck cancer that concurrent delivery of chemotherapy and radiation therapy provided improved local control and/or overall survival, but with increased acute toxicity [Citation3,Citation11–13]. The backbone of these chemotherapy regimens included cisplatin, given as either a singlet or doublet. Cisplatin is a heavy metal complex and has the ability to form intrastrand and interstrand cross-links, which inhibit DNA synthesis and result in cell-cycle arrest in the G2 phase of the cell cycle [Citation14,Citation15]. Cisplatin is known to be a radiosensitizer and is thought to enhance radiation cell kill via cisplatin-induced increased oxygenation of hypoxic cells as well as through an affinity for free electrons created during radiation therapy which disrupts sublethal damage repair mechanisms and leads to irreparable DNA damage [Citation16–18].
Cisplatin’s ability to form interstrand adducts with cell DNA is rapid acting and has a duration of up to 24 h after exposure. Beyond 24 h, the majority of DNA adducts will be gone [Citation15]. Based on these experiments, proposed mechanisms of radiosensitization, and pharmacokinetics of cisplatin, it has been presumed that cisplatin should be delivered prior to irradiation to maximize its additive effect. As discussed earlier, this is not always logistically possible and that notion has been reflected in the protocols of the prospective human trials investigating concurrent chemotherapy and radiation therapy for head and neck cancer, where chemotherapy may be given at a preferred but not mandated time point in relation to the initiation of radiotherapy. Some guidance on the temporal sequencing of these modalities has been demonstrated through in vitro studies, one of which examined single and split dose irradiation after exposure of platinum chemotherapy in a squamous cell carcinoma cell line [Citation19]. This study found that there was no potentiating effect if the drug was administered 24 h prior to irradiation and the maximum effect was noted if the drug compounds were present at the time of irradiation [Citation19]. One review summarized the available in vivo data as supporting drug delivery to be given a short time before irradiation as the most effective method and therefore recommended exploitation of this knowledge clinically [Citation20]. Our study appears to support this notion with improved 3-year LRC for patients who started chemotherapy first.
However, another concept that must be taken into consideration is the pharmacokinetics of cisplatin in human subjects. One study measured plasma concentration at various time points after cisplatin infusion and found the highest concentration at the end of the infusion with significant decline after 1 h regardless of the dose used [Citation21]. To take advantage of this, multiple early trials utilized low-dose daily or weekly concurrent cisplatin regimens [Citation22,Citation23]. Interestingly, two different studies by Jeremic et al. used different administration schedules of low-dose daily cisplatin: one in which cisplatin was given 30 min before and another in which cisplatin was administered 3–4 h after irradiation [Citation22,Citation24]. Similar outcomes were identified in both of these studies, despite chemotherapy being administered at differing timeframes surrounding irradiation. These data may support the finding that DNA-cisplatin adducts linger up to 24 h after cisplatin administration.
Knowledge about the basic science and pharmacokinetics in utilization of cisplatin has informed the current guidelines within modern prospective trials. An example of this can be identified in the current NRG trials investigating concurrent chemotherapy and radiation therapy where cisplatin infusion should be given within 24 h of irradiation (NCT03952585). Despite a benefit when chemotherapy is initiated prior to radiotherapy in LRC, our study did not find a difference in outcomes based on stratification of other timing sequences including the modalities starting on the same day or not, within 24 h or not, or certain days of the week. Though medical oncologists and radiation oncologists should seek to start chemotherapy prior to radiotherapy and ideally on the same day or at least within 24 h of each other, our study does not support that the inability to sequence these two modalities precisely will impact progression-free or overall survival outcomes, especially when delays in initiating treatment may have a detrimental impact outcomes [Citation25,Citation26].
This study is limited due to its retrospective nature and the results reported based on data abstracted from the medical record. Therefore, it is prone to patient selection bias and loss of patient follow up. The study is limited by its smaller sample size, which may have prevented identification of differences in survival after stratification of the patients into smaller comparison groups based on the timing of start of the chemotherapy and radiation therapy. Despite these limitations, this study offers further understanding how the timing of both chemotherapy and radiotherapy may impact patient outcomes in patients treated with definitive chemoradiation for head and neck cancer.
Conclusion
Starting chemotherapy prior to radiation therapy improves locoregional control. This improved LRC did not carry over to improved DS, PFS, or OS. Further prospective studies are warranted to confirm the ideal timing in the initiation of chemotherapy and radiation therapy in the use of definitive chemoradiation for patients with head and neck cancer.
Supplemental Material
Download TIFF Image (637.6 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, Cole R Steber, upon reasonable request.
Additional information
Funding
References
- Cancer Facts & Figures 2019. American Cancer Society. 2019.
- Creamer RM, Wang TW, Babb S, et al. Tobacco product use and cessation indicators among adults — United States, 2018. Morb Mortal Wkly Rep. 2019;68(45):1013–1019.
- Al-Sarraf M, Pajak TF, Marcial VA, et al. Concurrent radiotherapy and chemotherapy with cisplatin in inoperable squamous cell carcinoma of the head and neck. An RTOG Study. Cancer. 1987;59(2):259–265.
- Marcial VA, Pajak TF, Mohiuddin M, et al. Concomitant cisplatin chemotherapy and radiotherapy in advanced mucosal squamous cell carcinoma of the head and neck. Long-term results of the Radiation Therapy Oncology Group study 81-17. Cancer. 1990;66(9):1861–1868.
- El-Sayed S, Nelson N. Adjuvant and adjunctive chemotherapy in the management of squamous cell carcinoma of the head and neck region. A meta-analysis of prospective and randomized trials. J Clin Oncol. 1996;14(3):838–847.
- Pignon JP, le Maître A, Maillard E, et al. Journal Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92(1):4–14.
- Blanchard P, Baujat B, Holostenco V, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): a comprehensive analysis by tumour site. Radiother Oncol. 2011;100(1):33–40.
- Szturz P, Wouters K, Kiyota N, et al. Weekly low-dose versus three-weekly high-dose cisplatin for concurrent chemoradiation in locoregionally advanced non-nasopharyngeal head and neck cancer: a systematic review and meta-analysis of aggregate data. Oncologist. 2017;22(9):1056–1066.
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)-a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381.
- Nagatsuka M, Hughes RT, Shenker RF, et al. Omitting elective irradiation of the contralateral retropharyngeal nodes in oropharyngeal squamous cell carcinoma treated with intensity-modulated radiotherapy. Cureus. 2019;11(1):e3825.
- Adelstein DJ, Li Y, Adams GL, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol. 2003;21(1):92–98.
- Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized intergroup study 0099. J Clin Oncol. 1998;16(4):1310–1317.
- Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349(22):2091–2098.
- Prestayko A, Crooke S, Carter S. Cisplatin-current status and new developments. New York (NY): Academic Press; 1980.
- Marcu L, van Doorn T, Olver I. Cisplatin and radiotherapy in the treatment of locally advanced head and neck cancer - a review of their cooperation. Acta Oncol. 2003;42(4):315–325.
- Chu E, DeVita VT. Physicians’ cancer chemotherapy drug manual 2015. Burlington (MA): Jones & Bartlett Publishers; 2014.
- Yan RD, Durand RE. The response of hypoxic cells in SCCVII murine tumors to treatment with cisplatin and x rays. Int J Radiat Oncol Biol Phys. 1991;20(2):271–274.
- Sharma VM, Wilson WR. Radiosensitization of advanced squamous cell carcinoma of the head and neck with cisplatin during concomitant radiation therapy. Eur Arch Otorhinolaryngol. 1999;256(9):462–465.
- Schwachöfer JH, Crooijmans RP, Hoogenhout J, et al. Effectiveness in inhibition of recovery of cell survival by cisplatin and carboplatin: influence of treatment sequence. Int J Radiat Oncol Biol Phys. 1991;20(6):1235–1241.
- Dewit L. Combined treatment of radiation and cisdiamminedichloroplatinum (II): a review of experimental and clinical data. Int J Radiat Oncol Biol Phys. 1987;13(3):403–426.
- Rajkumar P, Mathew BS, Das S, et al. Cisplatin concentrations in long and short duration infusion: implications for the optimal time of radiation delivery. J Clin Diagn Res. 2016;10(7):XC01–XC4.
- Jeremic B, Shibamoto Y, Stanisavljevic B, et al. Radiation therapy alone or with concurrent low-dose daily either cisplatin or carboplatin in locally advanced unresectable squamous cell carcinoma of the head and neck: a prospective randomized trial. Radiother Oncol. 1997;43(1):29–37.
- Serin M, Erkal HS, Cakmak A. Radiation therapy and concurrent cisplatin in management of locoregionally advanced nasopharyngeal carcinomas. Acta Oncol. 1999;38(8):1031–1035.
- Jeremic B, Shibamoto Y, Milicic B, et al. Hyperfractionated radiation therapy with or without concurrent low-dose daily cisplatin in locally advanced squamous cell carcinoma of the head and neck: a prospective randomized trial. J Clin Oncol. 2000;18(7):1458–1464.
- Lauritzen BB, Jensen JS, Grønhøj C, et al. Impact of delay in diagnosis and treatment-initiation on disease stage and survival in oral cavity cancer: a systematic review. Acta Oncol. 2021;60(9):1083–1090.
- Huang J, Barbera L, Brouwers M, et al. Does delay in starting treatment affect the outcomes of radiotherapy? A systematic review. J Clin Oncol. 2003;21(3):555–563.