Abstract
Background
Nonepithelial ovarian cancer (NEOC) represents a wide variety of rare tumors. They are often diagnosed at an early stage and have a good prognosis compared to epithelial ovarian cancer. In the Nordic countries, the total annual number of patients diagnosed with ovarian cancer, Fallopian tube cancer or primary peritoneal carcinoma (hereafter ovarian cancer) was 2281 in 2014–2018, of which 3–10% were NEOC. International guidelines for diagnosis, treatment and follow-up have been developed. We present the results of a survey, aiming at clarifying current clinical practice in the Nordic countries.
Material and Methods
Between 09.2020 and 02.2021 a 33-question electronic survey was distributed to 22 hospitals in Finland, Sweden, Norway, Iceland and Denmark via the Nordic Society of Gynecological Oncology (NSGO) National Representatives. Data were collected in a secure web-based software platform. The questionnaire focused on demographics, diagnosis, treatment and follow-up programs.
Results
Twenty-one (95,4%) centers completed the survey. A total of 155 annual new NEOC cases treated in the Nordic countries were reported, corresponding to approximately 7% of all ovarian cancer cases. Most centers measured some or all of the recommended biomarkers routinely. Vaginal ultrasound and computed tomography (CT) were the preferred imaging modalities. The majority of centers conducted multidisciplinary team (MDT) meetings. The primary reported treatment was surgery. In 65% of centers, lymph node dissection was only performed in cases with suspicious lymph nodes. Surveillance was usually offered > four years.
Discussion
Despite, the presence of clinical European guidelines, variation in the current clinical practice amongst participating centers adhering to national guidelines was observed. Prospective clinical national research programs are sparse, and an enhanced cooperation in the Nordic countries toward development of a Nordic guideline and database is highly warranted and a prerequisite for future research, preferably in cooperation with the larger international groups.
Background
Nonepithelial ovarian cancer (NEOC) is a relatively rare condition [Citation1] comprising around 3–10% [Citation2,Citation3] of all malignant ovarian tumors. NEOC represents a wide variation of tumors. Roughly, the non-epithelial ovarian cancers can be divided into germ cell tumors (GCT) and sex cord-stromal tumors (SCST). GCT are primarily diagnosed in adolescents and younger women. SCST are a heterogeneous group presenting over a wider range of ages. The initial symptoms are pelvic pain and pelvic pressure. SCST are often associated with endocrine symptoms [Citation3,Citation4] and consequently biomarkers are used in the diagnostic procedures in combination with different imaging procedures. When treating adolescents or younger women with a NEOC, it is crucial to offer fertility-sparing surgery, whenever possible [Citation3,Citation5].
Due to the rarity of NEOC, the broad age distribution and histological heterogeneity, it has been difficult to monitor diagnosis, treatment and surveillance. For the same reasons, evidence and clinical practice NEOC guidelines are primarily based on retrospective data and case reports. International initiatives to develop guidelines to support the diagnosis, treatment and surveillance of NEOC have been made. In 2018, the European Society for Medical Oncology (ESMO) published their first clinical guideline on the subject [Citation3]. In 2019, the European Society of Gynecological Oncology (ESGO) and the European Society for Pediatric Oncology (SIOPE) published another guideline focusing on adolescents and young women [Citation5]. Apart from European societies, the National Comprehensive Cancer Network (NCCN) has published clinical practice guidelines for NEOC, as part of a general guideline for management of patients with ovarian cancer [Citation6].
In the Nordic countries, the annual number of ovarian cancers was 2281 in 2014–2018 [Citation7], which would correlate to approximately 68 to 228 (3–10%) annual cases of NEOC. Although a preliminary diagnosis is usually determined or suspected in local departments, the final diagnosis, treatment and surveillance of gynecological cancer are in the Nordic countries centralized to the Gynecology Oncology Centers specialized in surgical and oncological treatment. Despite this, detailed information regarding management of women with NEOC is lacking. To elaborate on this knowledge gap, a questionnaire-based electronic survey was created in cooperation with the Surgical Subcommittee of the Nordic Society of Gynecological Oncology (NSGO). The main focus was to evaluate the current clinical management of NEOC, most specifically SCST and GCT, in the Nordic countries, and to clarify if there is consensus in the clinical management and focus on prospective research in the field.
Material and methods
In September 2020, a 33-question electronic survey was distributed to NSGO Surgical Subcommittee National Representatives in Norway, Sweden, Finland, Iceland and Denmark. The questionnaire was also sent to key contacts in gynecological oncology and medical oncology departments in 22 centers: five centers in Denmark, five in Finland, one in Iceland, four in Norway, and seven in Sweden. In total, six reminders were sent by e-mail from September 2020 to February 2021.
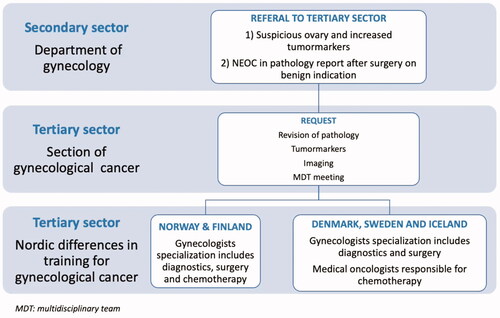
This distribution reflects the total population, since treatment of gynecological cancer in the Nordic countries is centralized to these specific centers. The treatment pathway and differences in specialization is illustrated in .
Figure 1. Pathway for diagnostics and treatment of patients treated for non-epithelial ovarian cancer (NEOC) and differences in training for gynecological cancer in the Nordic countries.
The survey was created and distributed through the Research Electronic Data Capture (REDCap) database hosted at OPEN in the Region of Southern Denmark. REDCap is a secure web-based software platform designed to support data capture for research studies [Citation8,Citation9].
The questions were grouped in different topics to capture information regarding demographics, the routine use of tumor markers and hormonal status pre- and post-operatively, imaging diagnostic tools, primary treatment including information of surgical management at the time of diagnosis, use of adjuvant therapy, existence of national guidelines, participation in clinical trials and finally information regarding surveillance. Questions referred to diagnostic work-up, treatment and surveillance of NEOC, focusing on the most frequent subtypes SCST and GCT.
Ethics
This study was an electronic survey sent to specialists in the Nordic countries and no person identification was relevant/possible. Therefore, no ethical committee approval was necessary according to national regulations.
Results
Physicians from 21 of 22 (95,4%) Nordic Gynecological Oncology Centers completed the survey. The survey was answered by an oncological gynecologist in 17 (81%) of 21 centers, the remaining four by a specialist from the Department of Medical Oncology. One center only partially completed the survey and was excluded from analysis.
Incidence
Overall, the centers reported 155 annual new cases of NEOC diagnosed in the Nordic countries. The median annual number of new NEOC cases per center was 5.5 (range 2–15.3); For SGST the median number was 3 (range 0.9–12) and for GCT 3 (range 1–5). Eight of the centers have a joint Department for Oncology and Gynecology, whereas the remaining centers have two separate departments for Oncology and Gynecology, respectively. The section of gynecological cancer within the Department of Obstetrics and Gynecology is responsible for the diagnostic part in all 21 centers and in three of these centers in collaboration with the Department of Pediatric Oncology.
Diagnosis
Diagnostic work-up is depicted in . When asked regarding routine blood tests as part of diagnostic work-up, all centers reported that cancer antigen 125 (CA125) was evaluated prior to surgery. The majority of centers also measured alpha-fetoprotein (AFP) (86%), beta-human chorionic gonadotropin (β-hCG) (86%), lactate dehydrogenase (LDH) (81%), Inhibin B (76%) and anti-Müllerian hormone (AMH) (71%) as part of the diagnostic work-up (). The preferred initial imaging modality was transvaginal ultrasound. In 16 of 21 centers computerized tomography (CT) was routine imaging, and if CT was not the routine imaging positron emission tomography and CT (PET-CT) was performed. In 81% of centers imaging was presented at a multidisciplinary team (MDT) meeting to ensure optimal initial therapy. In all centers, treatment was performed in accordance with a national guideline. Only 14% of the centers reported having a specific research program for NEOC.
Table 1. The current clinical practice in non-epithelial ovarian cancer (NEOC) in the Nordic Gynecological Oncology Centers based on a survey from the Surgical Subcommittee of the Nordic Society of Gynecological Oncology (NSGO).
Treatment
The initial treatment plan () was carried out at either the Department of Obstetrics and Gynecology, Department of Pediatric Oncology and/or the Department of Oncology. However, the section of gynecological cancer within the Department of Obstetrics and Gynecology was reported to be responsible for the initial treatment in 76% of the centers. Surgery was the preferred initial modality in almost all patients. For patients diagnosed with SCST surgery was the initial treatment modality in 100% and for patients with GCT in 95%. The majority of the centers (65% for both CGT and SCST) performed nodal lymph dissection only if abnormal lymph nodes were observed.
Table 2. Treatment of patients with nonepithelial ovarian cancer (NEOC) in the Nordic countries.
Follow-up
Women were offered long-term (>four years) standard surveillance after treatment in 19 of 21 centers (90%, ). Standard surveillance most frequently included clinical evaluation, ultrasound and tumor markers in both SCST and GCT (62% and 71% of centers, respectively).
Table 3. Follow-up of patients treated for nonepithelial ovarian cancer (NEOC) in the Nordic countries.
Discussion
Rare cancers have an annual incidence rate of ≤6/100.000 [Citation1], accounting for 24% of all cancers diagnosed in the EU during 2000–2007 [Citation10]. NEOC are rare ovarian cancers (0.25/100.000) [Citation10]; the two most frequent tumor types are GCT and SCST.
The GCT comprise the following subgroups; dysgerminoma and immature teratoma as the most common types (70%) and rarer types are yolk sac tumor, embryonal carcinoma, nongestational choriocarcinomas and mixed germ cell tumors. These tumors arise in the ovaries in girls and younger women [Citation3,Citation5].
The SCST are a heterogeneous group, comprising juvenile granulosa cell tumors, Sertoli cell tumors and Sertoli Leydig cell tumors in girls and younger women, and granulosa cell tumors and thecomas in peri- and postmenopausal women, among others. The initial symptoms in case of NEOC are often pelvic pain, pelvic pressure and irregular menstrual bleeding [Citation3].
summarizes the recommendations for diagnosis, treatment and follow-up according to ESMO Clinical Practice Guideline [Citation3] and NCCN guidelines [Citation5]. The overview is not simple and some differences between the ESMO and NCCN practices are seen, which is an obvious reflection of the complexity and sparse evidence in the field.
Table 4. Overview; Clinical Practice Guidelines for diagnosis, treatment and follow-up for non-epithelial ovarian cancer (NEOC) according to the National Comprehensive Cancer Network (NCCN) and the European Society for medical Oncology (ESMO).
Tumor markers should be measured in all young patients who present with a pelvic mass [Citation11,Citation12]. In this survey, the departments only applied all markers from 19–100% of patients before surgery, and from 19-81% at surveillance. As a minimum in the diagnostic work-up, CA125 ought to be supplemented with AFP, β-hCG, LDH, Inhibin B and AMH [Citation3]. In surveillance, these biomarkers can be differentiated as follows: AFP, β-hCG, LDH in GCT’s and Inhibin B and AMH in SCST. The survey did not measure this differentiation.
All departments have reported to perform transvaginal ultrasound as part of the diagnostic work-up, this answer reflects that this is a routine examination in the gynecological clinical practice, but it is very likely not performed in the youngest patients diagnosed before sexual debut. Furthermore, either CT or PET-CT was performed in all centers.
Surprisingly, not all patients were discussed at an MDT meeting, and only 81% of the performed imaging modality was presented at the meeting. The strength of the MDT meeting is that the treatment plan for the rare disease is discussed and adopted by all specialties involved. Moreover, the structure of the department differs as some departments are gynecological, some oncological and, in some hospitals the departments are merged; these differences make the MDT meeting even more important. For the diagnostic workup, the combination of biomarkers and imaging of pelvis, abdomen and thorax are essential. The variation in the preferred imaging modality in the clinical practice in the Nordic countries and the different possibilities in recommendations from ESMO and NCCN is a reflection of the technical availability in different settings. A consideration which is an essential part of the work when elaborating national and international recommendations.
Surgery is the primary treatment modality in the vast majority of cases. In general, NEOC have a good prognosis, and when presenting in early stages in young women, these can be candidates for fertility-sparing surgery without impeding oncological outcomes [Citation13]. Fertility-sparing treatment in children and young women includes a surgical approach with unilateral salpingo-oophorectomy and comprehensive surgical staging (cytology, peritoneal biopsies and omental biopsy). This can be offered safely to patients with GCT, even in advanced stages. Patients with SCST can be treated with fertility-sparing surgery, in selected cases, with FIGO stage IA to IC3 after comprehensive counseling [Citation13]. In older women, bilateral salpingo-oophorectomy, hysterectomy and comprehensive surgical staging is recommended [Citation14].
The reason for the different advice for fertility-sparing treatment in the case of GCT and SCST is the difference in chemo-sensitivity. The GCT’s are highly chemo-sensitive, and FIGO stage ≥ IC and tumor recurrence are offered chemotherapy [Citation4]; furthermore, in selected cases, oncological treatment followed by salvage surgery might be an option. On the contrary, SCST’s are not very chemo-sensitive and the preferred treatment in the Nordic countries in both primary and recurrent cases is therefore surgery.
There is a wide variation in the surgical approach especially in regards to lymph node dissection, and a more uniform attitude toward this procedure is desirable, as 10% perform systematic lymph node dissection, and 65% remove macroscopic suspicious lymph nodes. Adherence to the ESMO guideline would be desirable to reduce the side effects of systematic lymph node dissection without deterioration of survival [Citation3]. In the ESMO guideline, resection of lymph nodes is reserved to rare recurrent cases of GCT and is not recommended in SCGT [Citation3]. Opposite to this, the NCCN guideline has a more extensive approach toward lymph node dissection. In GCT’s stage IA-IIA full pelvic and paraaortic lymph node resection is preferred in accordance with epithelial ovarian cancer treatment and in SCGT lymph node dissection can be omitted if the tumor is grossly confined to the ovaries [Citation5].
There is an agreement toward surveillance with clinical examination, ultrasound and tumor markers. In the present survey, most Nordic centers offered surveillance for more than four years, but not more than five years which was also a possible answer. In the ESMO and NCCN recommendations, the surveillance is close in the first two years and described as lifelong for the majority of cases (see ). In all three settings, there is a variation in the intervals for and the use of imaging modalities in the follow-up.
Data collected in this survey describes an incidence of 7% of NEOC in centralized departments of 21 Nordic hospitals, similar to the reported incidence in previous studies. The majority of centers reported that they adhere to a national guideline, based on the current clinical practice recommendations presented in 2018 by ESMO, regarding diagnostic work-up (biomarkers, imaging modality and pathology review), treatment (regarding primary approach and nodal dissection) and surveillance [Citation3].
Thus, based on the responses in this survey, we conclude that it will be possible to optimize and streamline the current clinical practice concerning all the three aspects.
For the reason that NEOC are rare tumors, conducting prospective trials in order to understand the biological behavior and best treatment approach is challenging. Even so, only 50% of the centers in the Nordic countries reported new cases to a national quality database and only 14% reported to have a national research program to study NEOC.
Recently, several initiatives for research in rare cancers have been introduced but the participation from the Nordic centers has not been uniform. ESGO and the European Network for Gynecological Oncological Trials Group (ENGOT) have established a research collaboration in rare gynecologic cancers [Citation15]. This has resulted in initiation of the first international clinical trial investigating the treatment of women with SGCT [Citation16]. In 2007, the European Commission financed the RARECARE project [Citation17] dedicated to Rare Cancers, represented by Finland, Iceland and Norway. In particular, the creation of the European Reference Networks (ERNs) in 2017 as a network for research and knowledge between healthcare providers and patient representatives, has succeeded in providing specialized healthcare for patients with a rare disease [Citation18]. The EURopean rare Adult CANcer network (EUR A CAN) is one of the 24 ERNs, dedicated to rare adult solid cancers, where centers from Denmark, Finland, Norway and Sweden are participating [Citation19]. In the field of gynecologic cancer research, another European network, GYNOCARE, an EU funded program, was created to serve as a network covering, for example, basic research on rare gynecological cancer, biobanking, and legal requirements for international clinical trials [Citation18]. GYNOCARE is part of the European Collaboration in Science and Technology (COST-CA18117 – European Network for Gynecological Rare Cancer Research) [Citation20] focusing on the development of new approaches to improve the diagnosis and treatment of rare gynecological tumors. Although all the Nordic countries are currently members of COST, only Denmark and Norway are active participants in GYNOCARE.
The strength of our study is the high number of departments replying to the questionnaire. The limitation is that the questionnaire is exploring some of the rarest diseases, which are pooled as NEOC despite there is a wide variation within the different histological types.
Although data collected in our survey demonstrated a high degree of standardized management of NEOC patients in the Nordic countries, differences are still present in the current clinical practice for diagnostic work-up, treatment and surveillance, and there is a potential for optimization in the Nordic countries. We did not question the practices regarding fertility sparing surgery, which is an important issue to address for these patients. The Surgical Subcommittee of NSGO has the potential to provide a unique platform to initiate and strengthen the cooperation between centers in the Nordic countries and to take the initiative to develop a Nordic joint guideline. It will be extremely important to register all patients with a rare gynecological cancer in a Nordic database which could serve as a prime base for developing clinical trials in NEOC with a potential to elaborate strategies for the different subgroups and prospectively address the perspective of fertility sparing treatment. Additionally, the cooperation aims to contribute to the established European research collaborations e.g. ESGO and ENGOT.
Disclosure statement
The authors state to have no conflicts of interest.
Data availability statement
Data available on request from the authors (C. Daviu).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
References
- Information Network on Rare Cancers: RARECARENet. [Cited 2021 August 3] Avalaible from http://rarecarenet.istitutotumori.mi.it/rarecarenet/images/indicators/Incidence.pdf.
- Bennetsen AKK, Baandrup L, Aalborg GL, et al. Non-epithelial ovarian cancer in Denmark - Incidence and survival over nearly 40 years. Gynecol Oncol. 2020;157(3):693–699.
- Ray-Coquard I, Morice P, Lorusso D, et al. Non-epithelial ovarian cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv1–iv18.
- Dansk Gynaekologisk Cancer Gruppe. Visitation, diagnostik og opfølgning af ikke-epitelial ovariecancer.Kliniske retningslinjer på kraeftområdet, 2020. [Cited 2021 August 3]. Available from http://www.dgcg.dk/index.php/guidelines/ikke-epithelial-ovariecancer.
- Sessa C, Schneider DT, Planchamp F, et al. ESGO-SIOPE guidelines for the management of adolescents and young adults with non-epithelial ovarian cancers. Lancet Oncol. 2020;21(7):e360–e368.
- National Comprehensive Cancer Network. Ovarian Cancer Including Fallopian Tube Cancer and Primary Peritoneal Cancer (Version 1.2022). [Cited 2022. May 16]. Available from https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf.
- The Association of the Nordic Cancer Registries. Trends lines Nordcan, Dataversion 9.0. International Agency for Research on Cancer (IARC). [Cited 2021. July 27]. Available from https://nordcan.iarc.fr/en/dataviz/trends?sexes=2&cancers=220&key=total&group_populations=1&y.
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)-a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381.
- Harris PA, Taylor R, Minor BL, REDCap Consortium, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208.
- Gatta G, Capocaccia R, Botta L, et al. RARECAREnet working group. Burden and centralised treatment in Europe of rare tumours: results of RARECAREnet-a population-based study. Lancet Oncol. 2017;18(8):1022–1039.
- Shaaban A, Rezvani M, Elsayes KM, et al. Ovarian malignant germ cell tumors: cellular classification and clinical and imaging features. Radiographics. 2014;34(3):777–801.
- Boussios S, Zarkavelis G, Seraj E, et al. Non-epithelial ovarian cancer: elucidating uncommon Gynaecological Malignancies. Anticancer Res. 2016;36(10):5031–5042.
- Johansen G, Dahm-Kähler P, Staf C, et al. Fertility-sparing surgery for treatment of non-epithelial ovarian cancer: Oncological and reproductive outcomes in a prospective nationwide population-based cohort study. Gynecol Oncol. 2019;155(2):287–293.
- Medina-Franco H, Colonna-Márquez LE. Non-epithelial ovarian carcinoma: what is the optimal staging surgery? Chin Clin Oncol. 2020;9(4):50.
- Vergote I, Elser G, Votan B, member trial groups of the European Network of Gynaecological Trial groups (ENGOT), et al. Roadmap for the european network of Gynaecological Trial groups (ENGOT) Trials. Int J Gynecol Cancer. 2013;23(7):1339–1343.
- Ray-Coquard I, Harter P, Lorusso D, et al. Effect of weekly paclitaxel with or without bevacizumab on progression-free rate among patients with relapsed ovarian sex cord-stromal tumors: the ALIENOR/ENGOT-ov7 randomized clinical trial. JAMA Oncol. 2020;6(12):1923–1930.
- RARECAREnet leaflet: objectives. [Cited 2021 July 26]. Available from http://rarecarenet.istitutotumori.mi.it/rarecarenet/index.php/homepage/project/aims.
- Di Fiore R, Suleiman S, Ellul B, et al. GYNOCARE update: Modern strategies to improve diagnosis and treatment of rare gynecologic tumors-current challenges and future directions. Cancers (Basel). 2021;13(3):493.
- About EURACAN. [Cited 2021. August 3]. Available from https://euracan.eu/who-we-are/about-euracan/.
- COST: European Cooperation in Science and Technology. CA18117-European network for Gynecological Rare Cancer research: From Concept to cure. Participations. [Cited 2021 August 3]. Available from https://www.cost.eu/actions/CA18117/#tabs+Name:Parties. (Accessed August 13, 2021.
