Introduction
Radiotherapy (RT), often combined with chemotherapy (CRT), is the primary treatment strategy for squamous cell carcinoma of the anus (SCCA) [Citation1]. The treatment has been optimised through a series of phase II and III studies [Citation2–10] and is an effective and organ-preserving treatment strategy with around 80% of patients obtaining long-term tumour control [Citation11,Citation12]. The molecular biological features causing some SCCA tumours to be resistant to CRT are unknown. This is a central area for an investigation to ensure the best outcome for all SCCA patients. Hypoxia is a well-described biological feature that can influence radiosensitivity [Citation13]. Only limited attention has been paid to hypoxia in SCCA [Citation14], and no studies have yet investigated hypoxia in relation to treatment outcomes in SCCA. A 15-gene hypoxia classifier has been developed for head and neck squamous cell carcinomas (HNSCC) [Citation15]. The classifier categorises the tumours into a group of less hypoxic tumours and a group of more hypoxic tumours. The classifier was tested for its predictive value to identify patients who could benefit from treatment with the hypoxic radiosensitiser Nimorazole to increase loco-regional tumour control [Citation16]. Later, the classifier has been proved to have a prognostic value for the risk of loco-regional recurrence in both HNSCC [Citation15], uterine cervical SCC [Citation17] and SCC of the oesophagus [Citation18]. In a hypothesis-generating study, we investigated the expression of the 15 genes in the hypoxia classifier to examine if the gene expression was similar in SCCA as in the previously investigated SCC tumours. Furthermore, we compared the hypoxia classification to local tumour control in the SCCA cohort.
Methods
Diagnostic biopsies with verified invasive SCC from SCCA patients were evaluated for p16 status using immune histochemical staining with a cut-off for positivity of 70% [Citation19]. Hypoxia gene expression was measured using RNA extracts and quantified by RTqPCR, according to a previous described and validated in-house method [Citation15,Citation20].
All patients were included in the Danish Anal Cancer Group (DACG) study I, Plan-A, from 2015 to 2020. The study was approved by the Danish Data Protection Agency (2007-58-0010) and the Regional Ethical Committee (1-10-72-79-16).
Patients were staged according to the American Joint Committee on Cancer staging system version 7.0 and treated according to the Danish national guidelines [Citation21] with chemoradiotherapy. Patients with T1 or smaller T2 tumours (<4 cm) were considered for treatment with radiotherapy alone using a total dose of 64 Gy. Standard radiotherapy described doses were 60–64 Gy delivered in 30–32 fractions to the tumour and pathological lymph nodes. The elective clinical target volume was treated with 48–51.2 Gy in 30–32 fractions. Concomitant chemotherapy comprised cisplatin and fluorouracil or capecitabine alone or combined.
Local treatment failure was defined as treatment failure located in the pelvic region. Time of treatment failure was calculated from the day of end of treatment until the date of biopsy-proven SCCA.
Results
The mean expression levels for the 15 genes were compared between the 79 tissues from patients with SCCA and tissues from a representative cohort of 108 patients diagnosed with HNSCC and included in the DAHANCA studies 18 and 24 [Citation22,Citation23]. No samples were excluded due to technical issues. All 15 genes were expressed with comparable mean levels in the SCCA cohort as in the HNSCC cohort and the distance from the y = x line was within one standard deviation (SD) for all genes. The median SD for all genes in the SCCA samples was 0.26 (range 0.04–0.67) compared to 0.23 (range 0.03–1) for the HNSCC cohort. Results are outlined in .
Figure 1. Results on hypoxia classification of squamous cell carcinoma of the anus (SCCA) tissues using a 15-gene hypoxia classifier. A: Comparison between the mean expression level of each of the 15 genes between the SCCA samples on the x-axis and a representative head and neck squamous cell carcinoma (HNSCC) cohort on the y-axis. B: The sum scores for the 15 genes. A negative score categorises the tumour as less hypoxic, and a positive score categorises the tumour as more hypoxic. Each bar represents a case. For patients with local recurrence, the number of months from the end of treatment to the time for diagnosis of recurrence and tumour characteristics are measured in the table. C: Aalen-Johansen estimated correlation between hypoxia classification and local tumour control.
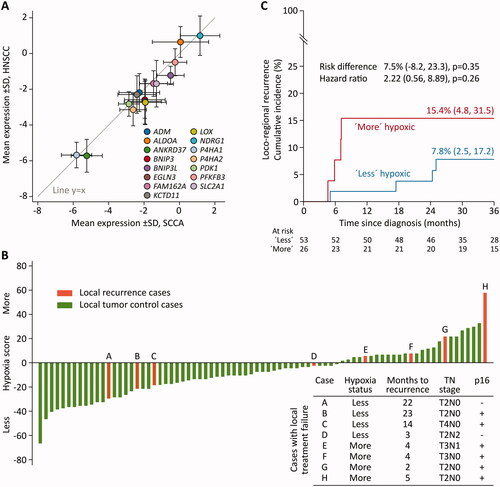
Classification of the SCCA tissues, as either less- or more hypoxic resulted in 53 (67%) less hypoxic tumours and 26 (33%) more hypoxic tumours.
Tumour stages for the SCCA cohort were 22% T1, 58% T2, 8% T3, and 10% T4, respectively. Lymph node involvement was found in 23%, and p16 overexpression was detected in 82% of the tumours. There was no difference between the more hypoxic and the less hypoxic tumours regarding patient- or tumour characteristics, including p16 status. Details are outlined in .
Table 1. Pretreatment Characteristics.
After a median of 46 months (range 9−70 months), eight patients had local treatment failure. The failures included both low stage (T2–T3, node-negative) and high stage (T3–T4, or node-positive) tumours and both p16-positive and p16-negative tumours. All the patients with local treatment failure received 60–64 Grey, and six of eight patients received concomitant chemotherapy.
Classification of the local treatment failures placed four tumours in the more hypoxic group and four tumours in the less hypoxic group. The fraction of local treatment failures in the more hypoxic group comprised four of 26 tumours (15%), compared to four of 53 tumours in the less hypoxic group (8%). Fishers exact test for equal variance between groups found no significant difference between classification and risk of local treatment failure, p = 0.4. The mean time from end of treatment to local treatment failure was 4 months (range 2−5 months) for patients with a more hypoxic tumour and 16 months (range 3−23 months) for patients with a less hypoxic tumour. The hypoxia scores are outlined in .
Using univariable Cox regression analysis and competing risk analysis by the Aalen-Johansen estimator, no significant difference was detected for increased risk for reduced local tumour control for patients with a more hypoxic tumour than for patients with a less hypoxic tumour. The hazard ratio was 2.22 (CI 0.56−8.89) p = 0.26 and risk difference was 7.5% (CI −8.2%−23.3%), p = 0.35, as illustrated in .
Discussion
The expression levels of each of the 15 genes found for the SCCA cohort is in line with the results on LACC [Citation17] and oesophageal SCC [Citation18]. All studies used the validated method with pre-defined cut-off values for the 15-gene hypoxia classifier developed by the DAHANCA group [Citation20]. Moreover, the distribution of around one-third of the tumours classified as more hypoxic and two-thirds of the tumours classified as less hypoxic is also in line with results found in the hypoxia studies on HNSCC, LACC and oesophageal SCC [Citation17,Citation18,Citation20]. The similar results on mean gene expression levels and distribution between less- and more hypoxic tumours indicates that the 15-gene hypoxia classifier may represent SCCs in general unrelated to the tumour site.
Evaluation of local treatment failures found a higher fraction of failures in the more hypoxic tumours compared to the less hypoxic tumour. Moreover, the failures in the more hypoxic tumours had a shorter mean time from end of treatment until diagnosis of the failure. Patients with local treatment failure within 6 months since end of treatment are often classified as having persistent disease and the results could indicate that the 15 gene hypoxia classifier could be predictive for the risk of persistent disease.
However, the low number of events and short follow-up time do not allow conclusions. A low number of events is often a limitation in SCCA studies due to the rarity of the disease and the high response rates to treatment. Only one other study has investigated hypoxia in SCCA [Citation14]. The study used oxygen enhanced T1 MRI scans to visualise the perfusion in the tumour, aiming at investigating the use of MRI imagine before and during RT to identify patients with a hypoxic tumour. Limitations in data completion from the 12 evaluated patients in the previous study did not allow for prognostic conclusions.
Conclusion
This hypothesis-generating study indicates that the 15-genes in the hypoxia classifier are expressed equally in SCCs from different sites. Therefore, it may be representative of SCCs in general. Hypoxia classification by the 15-gene classifier is a potential area for further investigation in SCCA with correlation to local treatment outcome. The low number of events in this dataset limits conclusions regarding the relationship between hypoxia and local tumour control in SCCA, but interesting trends were detected.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data not available due to legal restrictions.
Additional information
Funding
References
- Glynne-Jones R, Nilsson PJ, Aschele C, ESTRO, et al. Anal cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Radiother Oncol. 2014;111(3):330–339.
- Bartelink H, Roelofsen F, Bosset JF, et al. 103 Radiotherapy with concomitant chemotherapy superior to radiotherapy alone in the treatment of locally advanced anal cancer: Results of a phase III randomized trial of the EORTC radiotherapy and gastrointestinal tract cooperative groups. Int J Radiat Oncol. 1996;36(1):210.
- Flam M, John M, Pajak TF, et al. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: Results of a phase III randomized intergroup study. J Clin Oncol. 1996;14(9):2527–2539.
- Glynne-Jones R, Sebag-Montefiore D, Adams R, United Kingdom Coordinating Committee on Cancer Research Anal Cancer Trial Working Party, et al. Prognostic factors for recurrence and survival in anal cancer: Generating hypotheses from the mature outcomes of the first United Kingdom coordinating committee on cancer research anal cancer trial (ACT I). Cancer. 2013;119(4):748–755.
- James RD, Glynne-Jones R, Meadows HM, et al. Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): a randomised, phase 3, open-label, 2 × 2 factorial trial. Lancet Oncol. 2013;14(6):516–524.
- Kachnic LA, Winter K, Myerson RJ, et al. RTOG 0529: a phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluorouracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal. Int J Radiat Oncol Biol Phys. 2013;86(1):27–33.
- Northover JMA, Arnott SJ, Cunningham D, et al. Epidermoid anal cancer: Results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. Lancet. 1996;348(9034):1049–1054.
- Northover J, Glynne-Jones R, Sebag-Montefiore D, et al. Chemoradiation for the treatment of epidermoid anal cancer: 13-year follow-up of the first randomised UKCCCR anal cancer trial (ACT I). Br J Cancer. 2010;102(7):1123–1128.
- Peiffert D, Tournier-Rangeard L, Gérard JP, et al. Induction chemotherapy and dose intensification of the radiation boost in locally advanced anal canal carcinoma: final analysis of the randomized UNICANCER ACCORD 03 trial. J Clin Oncol. 2012;30(16):1941–1948.
- Rao S, Sclafani F, Eng C, et al. InterAACT: a multicentre open label randomised phase II advanced anal cancer trial of cisplatin (CDDP) plus 5-fluorouracil (5-FU) vs carboplatin (C) plus weekly paclitaxel (P) in patients (pts) with inoperable locally recurrent (ILR) or metastatic treatment. Ann Oncol. 2018;29(October):viii715–viii716.
- Guren MG, Aagnes B, Nygård M, et al. Rising incidence and improved survival of anal squamous cell carcinoma in Norway, 1987-2016. Clin Colorectal Cancer. 2019;18(1):e96–e103.
- Shakir R, Adams R, Cooper R, et al. Patterns and predictors of relapse following radical chemoradiation therapy delivered using intensity modulated radiation therapy with a simultaneous integrated boost in anal squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2020;106(2):329–339.
- Overgaard J, Horsman MR. Modification of Hypoxia-Induced radioresistance in tumors by the use of oxygen and sensitizers. Semin Radiat Oncol. 1996;6(1):10–21.
- Bluemke E, Bulte D, Bertrand A, et al. Oxygen-enhanced MRI MOLLI T1 mapping during chemoradiotherapy in anal squamous cell carcinoma. Clin Transl Radiat Oncol. 2020;22:44–49.
- Toustrup K, Sørensen BS, Metwally MAH, et al. Validation of a 15-gene hypoxia classifier in head and neck cancer for prospective use in clinical trials. Acta Oncol. 2016;55(9-10):1091–1098.
- Toustrup K, Sørensen BS, Lassen P, Danish Head and Neck Cancer Group (DAHANCA), et al. Gene expression classifier predicts for hypoxic modification of radiotherapy with nimorazole in squamous cell carcinomas of the head and neck. Radiother Oncol. 2012;102(1):122–129.
- Alsner J, Overgaard J, Tramm T, et al. Hypoxic gene expression is a prognostic factor for disease free survival in a cohort of locally advanced squamous cell cancer of the uterine cervix. Acta Oncol (Madr). 2021;0(0):1–7.
- Winther M, Alsner J, Tramm T, et al. Prognostic value of hypoxia-regulated gene expression in loco-regional gastroesophageal cancer. Acta Oncol. 2016;55(5):652–655.
- Serup-Hansen E, Linnemann D, Skovrider-Ruminski W, et al. Human papillomavirus genotyping and p16 expression as prognostic factors for patients with American joint committee on cancer stages I to III carcinoma of the anal canal. J Clin Oncol. 2014;32(17):1812–1817.
- Toustrup K, Sørensen BS, Nordsmark M, et al. Development of a hypoxia gene expression classifier with predictive impact for hypoxic modification of radiotherapy in head and neck cancer. Cancer Res. 2011;71(17):5923–5931.
- Danish Anal Cancer Group, clinical guidelines. doi:https://dacgnet.dk/kliniske-retningslinjer/.
- Saksø M, Mortensen LS, Primdahl H, et al. Influence of FAZA PET hypoxia and HPV-status for the outcome of head and neck squamous cell carcinoma (HNSCC) treated with radiotherapy: Long-term results from the DAHANCA 24 trial (NCT01017224). Radiother Oncol. 2020;151:126–133.
- Bentzen J, Toustrup K, Eriksen JG, et al. Locally advanced head and neck cancer treated with accelerated radiotherapy, the hypoxic modifier nimorazole and weekly cisplatin. Results from the DAHANCA 18 phase II study. Acta Oncol. 2015;54(7):1001–1007.
