Abstract
Background
Sepsis is the leading cause of admission to the intensive care unit (ICU) for cancer patients and survival rates have historically been low. The aims of this nationwide cohort study were to describe the characteristics and outcomes of cancer patients admitted to the ICU with sepsis compared with other sepsis patients requiring ICU admission.
Material and methods
This was a retrospective, observational study. All adult admissions to Icelandic ICUs during years 2006, 2008, 2010, 2012, 2014 and 2016 were screened for severe sepsis or septic shock by ACCP/SCCM criteria. Clinical characteristics and outcomes of sepsis patients with cancer were compared to those without cancer.
Results
In the study period, 235 of 971 (24%) patients admitted to Icelandic ICUs because of sepsis had cancer, most often a solid tumour (100), followed by metastatic tumours (69) and haematological malignancies (66). Infections were more often hospital-acquired in cancer patients (52%) than other sepsis patients (18%, p < 0.001) and sites of infections differed, with abdominal infections being most common in patients with solid and metastatic tumours but lungs and bloodstream infections in haematological malignancies. The length of stay in the ICU was shorter for sepsis patients with metastatic disease than other sepsis patients (2 vs. 4 days, p < 0.001) and they were more likely to have treatment limitations (52 vs. 19%, p < 0.05). Median survival of patients with metastatic disease was 19 days from ICU admission. The 28-day mortality (25%) of solid tumour patients was comparable to that of sepsis patients without cancer (20%, p < 0.001).
Conclusions
Cancer is a common comorbidity in patients admitted to the ICU with sepsis. The clinical presentation and outcome differs between cancer types. Individuals with metastatic cancer were unlikely to receive prolonged invasive ICU care treatment. Comparable short-term outcome was found for patients with solid tumours and no cancer.
Background
Cancer is a leading cause of death worldwide [Citation1], but mortality rates have been consistently falling in recent decades due to advances in treatment [Citation2]. There has even been a decline in the incidence of some tumours in the last decades, such as stomach and lung cancer in men [Citation3]. The overall incidence rate of all cancers between 2000 and 2017 was stable or declining slightly in males (-2.2% per year) but stable to increasing slightly in females (0.2% per year) [Citation4]. Due to improved survival and a change in demographics (age distribution) and risk factors (e.g. rising rates of obesity [Citation5]), the community prevalence of cancer is increasing, suggesting that more and more patients with cancer are likely to develop an indication for an ICU admission. In a recent study about 5% of all cancer patients were admitted to an intensive care unit (ICU) within two years of diagnosis [Citation6].
Historically, very high hospital mortality ratios have been reported for subgroups of cancer patients admitted to intensive care, such as 74% for haematological malignancy in 1988 [Citation7], 75% for multiple myeloma in 1990–1995 [Citation8] and 77% for solid tumours patients needing mechanical ventilation with an additional organ failure in 2007 [Citation9]. With a steady improvement in survival from cancer in the last decades [Citation10,Citation11], it is likely that more individuals with cancer are considered to potentially benefit from admission to the ICU for management of critical illness. Studies on cancer patients in the ICU have, however, been very heterogenous, with hospital mortality ratios ranging from 4.6 to 76.8% according to a recent literature review [Citation12]. In-hospital deaths are frequently preceded by decisions to forgo life-sustaining therapy, in situations where a meaningful recovery is highly unlikely, but practises vary between regions and hospitals [Citation13,Citation14].
Sepsis is a leading cause of acute admission of cancer patients to the ICU [Citation15,Citation16] and several studies have shown a trend towards decreasing mortality of cancer patients with sepsis [Citation17–20]. Many of the studies on sepsis in cancer patients have been conducted in ICUs in large cancer centres [Citation17,Citation19,Citation21,Citation22] and are often lacking a comparison group of sepsis patients without cancer [Citation19–21]. Additionally, some studies have been based on diagnostic codes [Citation18,Citation23] with an inherent risk of bias [Citation24].
The objectives of this study were to describe the characteristics and outcomes of cancer patients admitted to the ICU with sepsis and compare them with other sepsis patients from a clinically defined nationwide cohort.
Material and Methods
Study design and setting
The present study was a retrospective observational study of patients admitted to Icelandic ICUs because of sepsis. The only providers of intensive care in Iceland are Landspitali – The National University Hospital of Iceland in Reykjavik, which has 14 beds in two separate units, and Akureyri Hospital in Akureyri, which has three beds. The ICUs are ‘closed’ units and decisions regarding cancer patient admissions and limitations of treatment are made collectively between ICU physicians and haematologists/oncologists. All the ICUs are multi-disciplinary and there is a specialist in anaesthesia and intensive care in house 24-hours a day. The study protocol was approved by the National Bioethics Committee of Iceland (Case number: 16-088) and due to the observational nature of the study the need of informed consent was waived. The reporting of the study adhered to the STROBE guidelines for observational studies [Citation25].
Patient selection and data collection
Data on trends in the population incidence and outcome of sepsis requiring intensive care [Citation26] and data on the incidence and outcome of sepsis after elective surgery [Citation27] have been published from this patient cohort previously. All patients over 18 years of age admitted to Icelandic intensive care units in the calendar years 2006, 2008, 2010, 2012, 2014, and 2016 were screened for the presence of severe sepsis or septic shock on admission to the units. Patients were included every other year to reduce data collection resources. Modified 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions [Citation28] (Sepsis-2 criteria (Figure S1)) were used for patient inclusion but the more recent Sepsis-3 definitions [Citation29] were used post-hoc for classification of patients into sepsis and septic shock groups. Patients developing sepsis while staying in the intensive care unit for another reason were not included and re-admissions to the ICU during the same hospital stay for the same source of sepsis were not included again.
Figure 1. A flow chart over patients admitted to Icelandic ICUs with sepsis and the four groups compared in the present study.
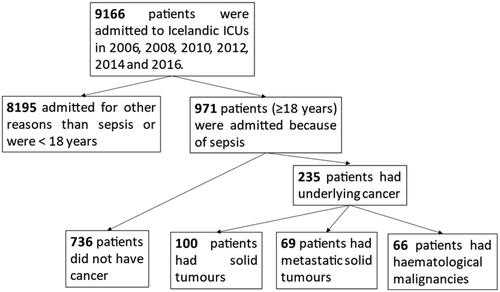
The following patient information was collected into a Microsoft Excel Database: Demographics and physiological data, laboratory and microbiology results and treatment administered. The APACHE II, [Citation30] SOFA, [Citation31] KDIGO [Citation32] and Charlson Comorbidity Index [Citation33] scoring systems were used to assess severity of illness and burden of underlying disease. Patients with active cancer diagnoses were divided into three groups:
Solid tumour without metastases, other than non-melanoma malignant neoplasm of skin. Patients were excluded if more than 5 years had passed from diagnosis.
Metastatic solid tumour.
Haematological malignancies, including acute and chronic myelogenous leukaemia, acute and chronic lymphocytic leukaemia, Hodgkin lymphoma, other lymphomas, lymphosarcoma, myeloma and Waldenström’s macroglobulinemia.
Six patients had two or more concurrent malignant diseases and were grouped according to what study authors jointly considered to be the clinically more important disease at the time of ICU admission. Neutropenia was defined as a neutrophil count <0.5 cells/mm3. Data on the prevalence of cancer in Iceland was acquired from Nordcan [Citation3]. Treatment limitation was defined as all decisions to forgo life sustaining therapy that were documented in patients’ charts before or during ICU admission and include: No cardiopulmonary resuscitation, no mechanical ventilation, no dialysis, no vasoactive therapy, no return to the ICU after discharge and transition to comfort care.
Microbiology
Only cultures taken within the first 48 h of ICU care, or in emergency departments and wards shortly before ICU admission were analysed in the study. Infections were considered hospital-acquired if they manifested more than 48 h after hospital admission. The initial empirical antimicrobial therapy was defined as insufficient if the cultured pathogens, which were considered clinically relevant, were resistant to the agents used by standard in vitro susceptibility tests. Cultures of commensal skin bacteria (e.g. coagulase-negative staphylococci) in blood were not considered pathogens unless cultured from repeated sets. Isolated findings of Candida spp. in respiratory or urine cultures were regarded as colonising organisms. Multi-resistant pathogens were defined as pathogens resistant to three or more classes of antibiotics. Microbiology findings were reviewed by a specialist in infectious diseases (MG).
Outcome variables
Information on demographics, aetiology of sepsis and outcome of cancer patients admitted to the ICU because of severe sepsis or septic shock were collected and analysed. Comparisons were made with data from patients admitted to the ICU with sepsis, but no malignancy, to evaluate differences between these groups.
Statistical analysis
Data are presented as medians with interquartile range [IQR] for continuous variables and as proportions for categorical variables. Incidence rates are reported with 95% confidence intervals. To analyse differences between two groups of patients (sepsis patients with or without cancer), Pearson’s Chi-squared test was used for categorical variables and Kruskal–Wallis test for continuous variables. When the four groups of sepsis patients (no cancer, solid tumour, metastatic tumour, haematological malignancy) were analysed, a Pearson’s Chi-squared test (categorical variables) or a Kruskal–Wallis test (continuous variables) between the four groups of patients was used. If these tests revealed a significant difference, a post hoc pairwise comparison with the Bonferroni correction for multiple tests was applied (alpha level 0.0083 (0.05/6)). Bonferroni corrected p-values are marked with padj. Survival is presented with Kaplan–Meier curves and median survival is presented with 95% confidence intervals. A comparison between the four groups of patients was performed with the log-rank (Mantel-Cox) test.
A propensity score matching was performed where patients with each of the three categories of cancer (solid tumour, metastasis, haematological) where matched at a 1:1 ratio with sepsis patients without cancer. The following parameters were matched: Age, gender, APACHE II score, SOFA score, modified Charlson comorbidity index and admission category. Group matching was assessed with standardised difference of matched parameters. A Cox proportional hazards model was constructed to analyse predictors of mortality in sepsis with age, sex, admission category, severity of illness (APACHE II and SOFA score), modified Charlson comorbidity index, body mass index, time period, insufficient empirical therapy, neutropenia and cancer included as co-variates. All p-values are two-tailed and a value of ≤0.05 was considered significant.
Missing data
When calculating the severity of illness scoring systems, if variables were missing they were assumed to have been within the normal range. In other analysis, patients with missing variables (or variable not applicable, for example, due to a pre-existing organ failure) were excluded from analysis of that variable and data from the remaining patients used. Missing values were frequent for body mass index (39%) and serum-lactate (13%). For other parameters reported in this study, missing values were less than 3%. For the Cox proportional hazards model, missing values were replaced with multiple imputation (100 iterations). Variables included in the imputation model were those included as predictors in the Cox model. Statistical analysis was performed using SPSS (IBM, version 26).
Results
The incidence of cancer in sepsis patients
In the study period (calendar years 2006, 2008, 2010, 2012, 2014, and 2016), a total of 971 patients were admitted to Icelandic ICUs because of severe sepsis or septic shock. Of these, 235 patients (24.2%, CI 21.5–27.0) had a concurrent active malignancy, most often a solid tumour (100), followed by metastatic solid tumour (69) and haematologic malignancies (66) ( and Table S1 for details of cancer diagnosis). The proportion of sepsis patients with underlying malignancy was larger in the latter half of the study period (2012–2016), 27.1%, (CI 23.3–31.2) compared with 20.8% (CI 17.3–24.9) in the first half (2006–2010) (p < 0.023). The 5-year prevalence of all cancers but non-melanoma skin cancers in Icelandic adults (≥20 years) increased during the study period from 17.9 to 18.5/1000 for women and from 16.9–18.3/1000 for men [Citation3]. The proportion of all adult cancer patients in Iceland admitted to intensive care with sepsis each year was 0.79% (CI 0.64–0.96) in 2006–2010 but 1.04% (CI 0.88–1.23) in 2012–2016 (p = 0.031).
Table 1. The characteristics, severity of illness and microbiology results from cancer patients admitted to the ICU with sepsis, compared with data from sepsis patients without cancer.
Clinical characteristics and infections
Patient characteristics are shown in . For all sepsis patient with cancer combined, the median age was 69 years [60–75], APACHE II score 22 [17–28] and SOFA score 9 [6–11]. Patients with solid tumours were older (70 years) than those with metastatic disease (65 years, padj=0.03)) and no malignancy (67 years, padj=0.03)). On a modified Charlson CI (points for the malignant disease itself removed) patients with metastatic disease had a lower severity of comorbid illness (2 (1–3)) than patients without cancer (3 (2–5), padj=0.001) or only solid tumour (3 (2–5), padj=0.001).
The severity of acute illness was highest in haematological patients with APACHE II score 26 vs. 20–21 in the other three groups (padj<0.001)) and SOFA score 10 vs. 7–8 in the other three groups (padj<0.001)). Admission categories differed between the groups of patients, with haematological patients most likely to be admitted from medical wards (64 vs. 17–38% in other groups, padj<0.05), patients with solid tumours most likely to be admitted from surgical wards (58% vs. 6–23% in other groups, padj<0.05) while sepsis patients without cancer were most likely to be admitted from emergency departments (55 vs. 25–39% in other groups, padj<0.05).
Data on sites of infections and pathogens are shown in . All cancer patients combined were more likely to have hospital-acquired infections than other sepsis patients, 52% (122/235) vs. 18% (131/736) (p < 0.001). Blood/endovascular infections were more common in haematological patients (21%) than solid tumour patients (2%, padj<0.05) and other sepsis patients (6%, padj<0.05). The incidence of insufficient empirical antimicrobial therapy was higher in patients with solid tumours (33%) than sepsis patients without cancer (16%, padj<0.05). The proportion of multi-resistant pathogens in the overall cohort was low, 2% (13/670)).
Treatment in the intensive care units
The duration of mechanical ventilation was shorter for patients with metastatic disease (2 days) than for sepsis patients without cancer (5 days, padj=0.014) and haematological patients (6.5 days, padj=0.049) (). The length of stay in the ICU was also shorter for patients with metastatic disease, 2 days, versus 4 days for other sepsis patients (padj<0.001). Decisions regarding limitations of treatment had been made before ICU admission in 6% (14/235) of cancer patients and 2% (17/736) in other sepsis patients (p = 0.006). For patients admitted without registered limitations, decisions to forgo one or more life sustaining therapy were made for 30% (70/235) of cancer patients and 17% (125/736) of other sepsis patients (p < 0.001) during the ICU stay. The first decision was made at a median of ICU day 1 (IQR 1–3) in cancer patients but at a median of ICU day 2 (IQR 1–6) in other sepsis patients (p = 0.14). A flow-chart of treatment limitations is presented in .
Figure 2. A flow chart depicting the number and timing of documented decisions to limit treatment in the group of patients admitted to Icelandic ICUs with sepsis along with patient mortality.
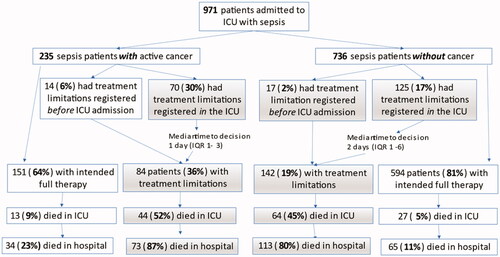
Table 2. The intensive care treatments administered to the four groups of sepsis patients and outcome.
Outcome
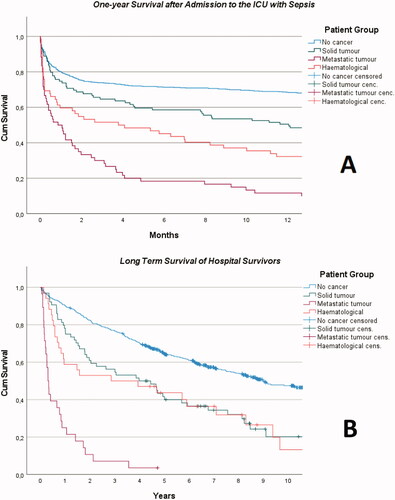
Mortality ratios are shown in and respectively. The ICU, hospital and one-year mortality ratios for all cancer patients with sepsis were 24% (57/234), 46% (107/233) and 67% (157/235), respectively. The median survival from ICU admission was 353 days (CI 74–632) for patients with solid tumours, 19 days (CI 4–34) for metastatic disease, 64 days (CI 0–194) for haematological malignancies but 4.7 years (CI 3.7–5.7) for other sepsis patients (p < 0.001). For hospital survivors, the median survival from hospital discharge was 3.9 years (CI 1.7–6.2) for patients with solid tumours, 91 days (CI 60–122) for metastatic disease, 2.5 years (CI 0–6.9) for haematological malignancies and 9.0 years (CI 7.2–10.7) for sepsis patients without cancer (p < 0.001). Of the cancer patients where a treatment limitation decision had been made 88% (74/84) died in hospital.
Figure 3. Kaplan–Meier curves for one-year survival of the four groups of sepsis patients (graph A) and long-term survival after hospital discharge (graph B).
In propensity score matched cohorts, there was no significant difference in the use of mechanical ventilation, length of stay, limitations of treatment or mortality between solid tumour patients and other sepsis patients (Table S2). Sepsis patients with metastatic disease remained less likely to receive mechanical ventilation, had a shorter length of stay, were more likely to receive limitations of treatment and had higher mortality ratios (hospital, 28-day and one-year) than the matched cohort of sepsis patients without cancer (Table S3). Sepsis patients with haematological disease were less likely to receive invasive mechanical ventilation and had a higher mortality (ICU, 28-day and one-year) than the matched cohort without cancer but there was no difference in the length of stay or the frequency of treatment limitations (Table S4). Survival of the three propensity score matched cohorts is presented in Figures S2–S4. Finally, a multivariable Cox proportional hazards model including age, comorbidity and severity of illness was constructed. Metastatic disease remained a strong predictor of mortality (HR 5.94 (95% CI 4.47-7.89)). Other factors associated with increased risk of mortality were age, medical admissions, APACHE II score, SOFA score, modified Charlson CI, inadequate empirical therapy, solid tumours and haematological malignancies (). Adjusted survival curves are presented in Figure S5.
Table 3. A multivariable Cox regression analysis of risk factors for mortality in patients admitted to ICUs with sepsis.
Discussion
In the present study, we found that a large proportion of patients admitted to intensive care units because of sepsis have an underlying malignant disease. Cancer patients with sepsis are a heterogenous group with variable sites of infections. In general, patients with metastatic disease had a short duration of stay and decisions to forgo further life-sustaining therapy were frequent. All groups of cancer patients with sepsis had reduced long-term survival compared with sepsis patients without cancer, but short-term survival ratios were comparable for patients with solid tumours.
In line with rising prevalence of cancer [Citation3], we found a slight increase in the proportion of sepsis patients with cancer from the first half (21%) to the latter half (27%) of the 11-year study period. These proportions are in the higher range of previous epidemiological studies on sepsis where 11% to 23% of patient cohorts have had underlying malignancy. Metastatic disease was present in 7% of our cohort of sepsis patients, which is also in the higher range of other reports (3–7%) [Citation34–36].
Although we did not include predetermined ICU admissions for postoperative observation following elective procedures, a large proportion (56%) of patients with solid tumours had recently undergone surgery and had postoperative sepsis. Major oncological procedures such as oesophagectomy, pancreatectomy, gastrectomy, cystectomy and colectomy are the elective operations associated with the highest rates of sepsis [Citation27,Citation37,Citation38]. Patients undergoing these procedures are frequently malnourished and may have received neo-adjuvant chemotherapy or radiotherapy, which are risk factors for postoperative sepsis [Citation37]. These patients had high rates of polymicrobial, abdominal infections with associated high risk of insufficient empirical antimicrobial therapy.
We found that the number and pattern of organ dysfunction was similar between the groups of cancer patients, apart from a higher incidence of coagulopathy in haematological patients. The frequent thrombocytopenia and immunosuppression in those patients likely explains the higher severity of illness scores (APACHE II and SOFA) compared with other sepsis patients. We did not see more frequent use of non-invasive ventilation (NIV) in haematological patients compared with other sepsis patients. Early initiation of NIV has been linked to reduced mortality in immunosuppressed patients [Citation39], but not confirmed in a later study [Citation40]. Our study was conducted before the use of high-flow nasal oxygen became widespread.
We found that patients with metastatic disease were younger than patients with solid tumours and had a lower severity of comorbid illness than other groups of sepsis patients. This illustrates the preceding selection of patients for admission into the ICU, although our high rates of metastatic disease in the whole sepsis cohort indicate that ICU admission policies are liberal in Iceland. The duration of mechanical ventilation was short for patients with metastatic diseases, 2 days, versus 5 days in sepsis patients without cancer. Decisions to forego invasive life sustaining therapy were common (52% of patients) and the hospital mortality ratios of patients with treatment limitations was high.
For all cancer patients with sepsis combined, we found an ICU-mortality ratio of 24%, which is lower than reported in recent studies on similar cohorts (41–53%) [Citation21,Citation22,Citation41,Citation42]. Icelandic hospitals do not have high-dependency units and all patients needing vasoactive support are admitted to an ICU, which might lead to inclusion of less severely ill patients into our study. The median APACHE II (22) and SOFA (9) were however comparable to those reported in similar studies [Citation22,Citation41]. Additionally, policies might differ between institutions regarding discharge from the ICU to wards for end-of-life care. Our hospital mortality ratio for all cancer patients with sepsis (46%) is in the lower range of previous reports (41–65%) [Citation19,Citation21,Citation22,Citation41–43] and the one-year ratio (67%) similar to others (62%) [Citation43].
It has been proposed that critically ill cancer patients with uncertain prognosis receive a time-limited trial in the ICU with full-code status before decisions to limit treatment are made [Citation9]. One argument for this being that mortality has been better predicted by the severity of organ failure, rather than the cancer characteristics in some studies [Citation16,Citation44]. A trial of five days has been proposed, although all patients who needed additional interventions (intubation, vasopressors or dialysis) after day three died in the study testing out this hypothesis [Citation9]. In a later study, a shorter one to four day trial is suggested for poor prognosis solid tumours [Citation45]. In our study, the median length of ICU stay for cancer patients of only 2–5 days suggests that if time limited trials were employed, they were generally short.
Only 6% of cancer patients had treatment decisions registered before ICU admission but an additional 30% received limitations during the ICU stay. Interestingly, the median time to first decision was only one day after ICU admission This indicates that the acute illness caused by sepsis acts as a trigger for treatment goal discussions. It could even suggest that some patients may have been inappropriately admitted to the ICU, where invasive therapy was considered futile very soon after admission. The time to treatment decisions in our study did not differ significantly between cancer patients and other sepsis patients but is shorter than the 2–4 days previously reported from observational studies [Citation14,Citation46].
The benefit of ICU admissions for patients no longer eligible to cancer treatment or with a very short life expectancy was recently described as limited in a recent consensus conference of the European Lung Cancer Working Party and the Groupe de Recherche Respiratoire en Réanimation Onco-Hématologique [Citation47]. Despite a short median survival of patients with metastatic cancer and sepsis (19 days) and a high one-year mortality, 29% of these patients were discharged back home after the ICU stay, and those discharged had a median survival of 91 days from hospital discharge. This argues that on a case-by case basis, a meaningful outcome can be reached with an ICU admission for patients with an otherwise incurable disease.
The strength of our study is the large, nationwide cohort of sepsis patients admitted to the ICU and data collection by detailed chart review instead of using administrative databases. Limitations include the small number of patients with cancers and since this study was limited to ICU patients, we lack information on the demographics and outcome of cancer patients with sepsis that were not considered eligible for ICU admission.
Conclusions
In this nationwide, clinically defined sepsis cohort, a quarter of the patients had underlying active cancer diagnosis, but the characteristics of sepsis and outcomes differed by groups of malignancies. Patients with solid tumours frequently had postoperative sepsis with similar short-term outcome as sepsis patients without cancer. The severity of illness on admission was highest in haematological patients, which along with patients with metastatic disease had reduced short- and long-term survival. The use of extended invasive ICU resources was limited in patients with metastatic cancer.
Supplemental Material
Download MS Word (115.6 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, SK, upon reasonable request.
Additional information
Funding
References
- CANCER TODAY. Data visualization tools for exploring the global cancer burden in 2020: World Health Organization; 2021 [cited 2021 Nov 11]. Available from: https://gco.iarc.fr/today/home.
- Brenner H, Gondos A, Arndt V. Recent major progress in long-term cancer patient survival disclosed by modeled period analysis. J Clin Oncol. 2007;25(22):3274–3280.
- Larønningen S, Ferlay J, Bray F, et al. NORDCAN: Cancer incidence, mortality, prevalence and survival in the Nordic countries, version 9.1 (27.09.2021). Association of the Nordic Cancer Registries. Cancer Registry of Norway. [cited 2022 Jun 19]. Available from: https://nordcan.iarc.fr/
- Islami F, Ward EM, Sung H, et al. Annual report to the nation on the status of cancer, part 1: National Cancer Statistics. J Natl Cancer Inst. 2021;113(12):1648–1669.
- Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6–10.
- Puxty K, McLoone P, Quasim T, et al. Risk of critical illness among patients with solid cancers: a population-based observational study. JAMA Oncol. 2015;1(8):1078–1085.
- Lloyd-Thomas AR, Wright I, Lister TA, et al. Prognosis of patients receiving intensive care for lifethreatening medical complications of haematological malignancy. Br Med J. 1988;296(6628):1025–1029.
- Peigne V, Rusinová K, Karlin L, et al. Continued survival gains in recent years among critically ill myeloma patients. Intensive Care Med. 2009;35(3):512–518.
- Lecuyer L, Chevret S, Thiery G, et al. The ICU trial: a new admission policy for cancer patients requiring mechanical ventilation. Crit Care Med. 2007;35(3):808–814.
- Shimabukuro-Vornhagen A, Böll B, Kochanek M, et al. Critical care of patients with cancer. CA Cancer J Clin. 2016;66(6):496–517.
- Azoulay E, Soares M, Darmon M, et al. Intensive care of the cancer patient: recent achievements and remaining challenges. Ann Intensive Care. 2011;1(1):5.
- Puxty K, McLoone P, Quasim T, et al. Survival in solid cancer patients following intensive care unit admission. Intensive Care Med. 2014;40(10):1409–1428.
- Guidet B, Flaatten H, Boumendil A, VIP1 Study Group, et al. Withholding or withdrawing of life-sustaining therapy in older adults (≥ 80 years) admitted to the intensive care unit. Intensive Care Med. 2018;44(7):1027–1038.
- Azoulay E, Metnitz B, Sprung CL, SAPS 3 investigators, et al. End-of-life practices in 282 intensive care units: data from the SAPS 3 database. Intensive Care Med. 2009;35(4):623–630.
- Hawari FI, Nazer LH, Addassi A, et al. Predictors of ICU admission in patients with cancer and the related characteristics and outcomes: a 5-year registry-based study. Crit Care Med. 2016;44(3):548–553.
- Soares M, Caruso P, Silva E, et al. Characteristics and outcomes of patients with cancer requiring admission to intensive care units: a prospective multicenter study. Crit Care Med. 2010;38(1):9–15.
- Cooper AJ, Keller SP, Chan C, et al. Improvements in sepsis-associated mortality in hospitalized patients with cancer versus those without cancer. A 12-year analysis using clinical data. Ann Am Thorac Soc. 2020;17(4):466–473.
- Danai PA, Moss M, Mannino DM, et al. The epidemiology of sepsis in patients with malignancy. Chest. 2006;129(6):1432–1440.
- Legrand M, Max A, Peigne V, et al. Survival in neutropenic patients with severe sepsis or septic shock. Crit Care Med. 2012;40(1):43–49.
- Pène F, Percheron S, Lemiale V, et al. Temporal changes in management and outcome of septic shock in patients with malignancies in the intensive care unit. Crit Care Med. 2008;36(3):690–696.
- Rosolem MM, Rabello LS, Lisboa T, et al. Critically ill patients with cancer and sepsis: clinical course and prognostic factors. J Crit Care. 2012;27(3):301–307.
- Awad WB, Nazer L, Elfarr S, et al. A 12-year study evaluating the outcomes and predictors of mortality in critically ill cancer patients admitted with septic shock. BMC Cancer. 2021;21(1):709.
- Hensley MK, Donnelly JP, Carlton EF, et al. Epidemiology and outcomes of cancer-related versus non-cancer-related sepsis hospitalizations. Crit Care Med. 2019;47(10):1310–1316.
- Rhee C, Klompas M. Sepsis trends: increasing incidence and decreasing mortality, or changing denominator? J Thorac Dis. 2020;12(Suppl 1):S89–s100.
- Vandenbroucke JP, von Elm E, Altman DG, STROBE Initiative, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. PLOS Med. 2007;4(10):e297.
- Vesteinsdottir E, Sigurdsson MI, Gottfredsson M, et al. Temporal trends in the epidemiology, management and outcome of sepsis — a nationwide observational study. Acta Anaesthesiol Scand. 2022;66(4):497–506.
- Vesteinsdottir E, Gottfredsson M, Blondal A, et al. Sepsis after elective surgery — Incidence, aetiology and outcome. Acta Anaesthesiol Scand. 2021;65:457–465.
- Levy MM, Fink MP, Marshall JC, SCCM/ESICM/ACCP/ATS/SIS, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med. 2003;31(4):1250–1256.
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801–810.
- Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829.
- Vincent JL, Moreno R, Takala J, On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine, et al. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22(7):707–710.
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–84.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Karlsson S, Varpula M, Ruokonen E, et al. Incidence, treatment, and outcome of severe sepsis in ICU-treated adults in Finland: the Finnsepsis study. Intensive Care Med. 2007;33(3):435–443.
- Ogura H, Gando S, Saitoh D, Japanese Association for Acute Medicine Sepsis Registry (JAAMSR) Study Group, et al. Epidemiology of severe sepsis in Japanese intensive care units: a prospective multicenter study. J Infect Chemother. 2014;20(3):157–162.
- Kadri SS, Rhee C, Strich JR, et al. Estimating ten-year trends in septic shock incidence and mortality in United States Academic Medical Centers using clinical data. Chest. 2017;151(2):278–285.
- Sood A, Abdollah F, Sammon JD, et al. Postoperative sepsis prediction in patients undergoing major cancer surgery. J Surg Res. 2017;209:60–69.
- Vogel TR, Dombrovskiy VY, Carson JL, et al. Postoperative sepsis in the United States. Ann Surg. 2010;252(6):1065–1071.
- Hilbert G, Gruson D, Vargas F, et al. Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acute respiratory failure. N Engl J Med. 2001;344(7):481–487.
- Lemiale V, Mokart D, Resche-Rigon M, Groupe de Recherche en Réanimation Respiratoire du patient d’Onco-Hématologie (GRRR-OH), et al. Effect of noninvasive ventilation vs oxygen therapy on mortality among immunocompromised patients with acute respiratory failure: a randomized clinical trial. JAMA. 2015;314(16):1711–1719.
- Torres VB, Azevedo LC, Silva UV, et al. Sepsis-associated outcomes in critically ill patients with malignancies. Ann Am Thorac Soc. 2015; 12(8):1185–1192.
- Zuber B, Tran TC, Aegerter P, et al. Impact of case volume on survival of septic shock in patients with malignancies. Crit Care Med. 2012;40(1):55–62.
- Man MY, Shum HP, Lam SM, et al. Effect of the underlying malignancy on critically ill septic patient’s outcome. Asia Pac J Clin Oncol. 2021. DOI:10.1111/ajco.13638
- Azoulay E, Moreau D, Alberti C, et al. Predictors of short-term mortality in critically ill patients with solid malignancies. Intensive Care Med. 2000;26(12):1817–1823.
- Shrime MG, Ferket BS, Scott DJ, et al. Time-limited trials of intensive care for critically ill patients with cancer: how long is long enough? JAMA Oncol. 2016;2(1):76–83.
- Poukkanen M, Vaara ST, Pettilä V, FINNAKI study group, et al. Acute kidney injury in patients with severe sepsis in Finnish Intensive Care Units. Acta Anaesthesiol Scand. 2013;57(7):863–872.
- Meert AP, Wittnebel S, Holbrechts S, Critically ill cancer patients consensus conference group, et al. Critically ill cancer patient’s resuscitation: a Belgian/French societies’ consensus conference. Intensive Care Med. 2021;47(10):1063–1077.
