Abstract
Background
Dose escalation for skull-based malignancies often presents risks to critical adjacent neural structures, including the brainstem. We report the incidence of brainstem toxicity following fractionated high-dose conformal proton therapy and associated dosimetric parameters.
Material and methods
We performed a single-institution review of patients with skull-base chordoma or chondrosarcoma who were treated with proton therapy between February 2007 and January 2020 on a prospective outcomes-tracking protocol. The primary endpoint was grade ≥2 brainstem toxicity. No patients received concurrent chemotherapy, and brainstem toxicity was censored for analysis if it coincided with local disease progression.
Results
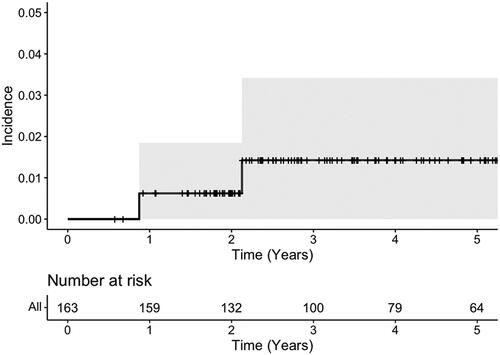
We analyzed 163 patients who received a minimum of 45 GyRBE to 0.03 cm3 of the brainstem. Patients were treated to a median total dose of 73.8 (range 64.5–74.4) GyRBE at 1.8 GyRBE per fraction with 17 patients undergoing twice-daily treatment at 1.2 GyRBE per fraction. With a median follow-up of 4 years, the 5-year cumulative incidence of grade ≥2 brainstem injury was 1.3% (95% CI 0.25–4.3%). There was one grade 2, one grade 3, and no grade 4 or 5 events, with all patients recovering function with medical management.
Conclusion
In delivering curative-intent radiotherapy for skull-base chordoma and chondrosarcoma in adults, small volumes of the brainstem can safely receive at least 64 GyRBE with minimal risk of serious brainstem injury.
Background
Chordoma and chondrosarcoma are rare sarcomas that can affect the bones of the skull base and require high doses of radiotherapy for oncologic control. These tumors often lie near sensitive neurovascular tissues and organs at risk (OARs). Of these OARs, the brainstem serves as the conduit between the cortical motor and sensory complexes to the peripheral nervous system. Injury to the brainstem can lead to life-threatening neurologic disorders including dysarthria, dysphagia, and even paralysis [Citation1,Citation2].
As National Cancer Database (NCDB) studies [Citation3,Citation4] have demonstrated that dose escalation above 70 Gy has been associated with increased overall survival in patients with chordoma and chondrosarcoma, the brainstem tolerance threshold used at centers specializing in therapy for this population typically exceeds dose tolerance limits used in cooperative group trials [Citation5–14]. Since much of the data used to develop brainstem constraint limitations were collected before the widespread availability of CT-based planning and highly conformal treatment delivery techniques, such as intensity-modulated radiotherapy and proton therapy, acceptable dose limits for patients with radioresistant skull-based malignancies remain unclear. Additional theoretical questions exist concerning variations in relative biologic effectiveness (RBE) within the distal Bragg peak, which could increase complications in adjacent normal tissues distal to the treatment field, specifically when delivering particle therapy.
With the goal of informing our understanding of radiotherapy-induced brainstem toxicity, the present series reports a dosimetric and outcomes evaluation of brainstem radionecrosis in adults following high-dose conformal proton radiotherapy.
Material and methods
We conducted a single-institution analysis of adult patients (age >21 years) with radiation-naïve skull-base chordoma or chondrosarcoma who were treated with proton therapy at the University of Florida in Jacksonville, FL on an institutional review board-approved prospective outcome tracking protocol between February of 2007 and January of 2021. Patients were included if they received a minimum of 45 GyRBE to 0.03 cm3 of the brainstem, under the assumption that doses below this threshold would not result in complications and inaccurately skew risk estimates. Treatment planning techniques, including simulation, patient immobilization, target delineation, and dose coverage goals and constraints, have been previously summarized [Citation12–14].
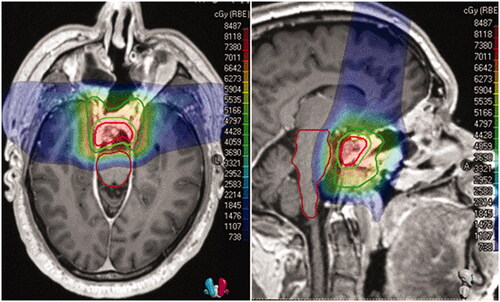
In regards to the brainstem OAR, to mitigate RBE uncertainties within the distal fall-off, the brainstem was within the spread out Bragg peak in the initial phase and blocked by apertures in the boost phase of those treated with a double-scattered (DS) approach. The total number of beams and beam placement was determined based on anatomy. If the target volume was directly anterior to the brainstem, then lateral anterior oblique fields and a superior vertex field were used. If a component of tumor had wrapped around the brainstem in a non-conformal geometry, the lateral obliques were matched to the lateral penumbra of a posterior beam in a 5-field patch-and-through technique for the DS proton plans or with multi-field optimization in those treated with pencil-beam scanning (PBS). Beam angles between treatment phases were deliberately offset by a minimum of 10° from the initial and reduction. In all cases, no more than 1 of the 3 initial fields could end on the brainstem and none within the boost. For those treated with PBS, Monte Carlo optimization with a 3-mm/3.5% independent beam robustness was applied. While no independent planning OAR volumes were used, robustness constraints were placed on the brainstem and specific spots were limited to 2 MU per spot. For limited cases, proximal Bragg Peak spots were allowed within the brainstem, while those at the distal range were placed 3 mm of the OAR. As clinical application of variable RBE modeling is an active area of investigation, the standard 1.1 factor to account for differences in RBE was applied. Daily image guidance was used in all cases, either kilovoltage orthogonal radiographs with DS or cone-beam computed tomography (CBCT) with PBS. A representative example 3-field pencil-beam technique with a colorwash dose distribution is noted in .
Figure 1. Colorwash dose distribution. The gross tumor volume (GTV) is in red, the planning target volume (PTV) to 50.4GyRBE is in forest green, to 73.8 GyRBE is in lime green, and the brainstem is in maroon.
Our current institutional guidelines recommend limiting the dose to 0.1 cm3 <64 GyRBE (D0.1 cc <64 GyRBE) to the surface of the brainstem and <60 GyRBE to the brainstem core (D0.1 cc <60 GyRBE). The brainstem core is defined as a 3-mm anisotropic contraction in all directions except superior-inferior. Guidelines were achieved in nearly all cases; if dose limits were exceeded, it was performed carefully under informed consent in patients with gross disease abutting the surface of the brainstem. For each patient, dose-volume histogram (DVH) data were extracted using verified contours.
Complication reporting and evaluation
Complications were defined per the definition for brainstem complications within the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE), version 5.0. After completion of radiotherapy, patients were recommended to engage in standard oncologic follow-up evaluation with either our team or a local oncologist on an annual basis, if not more frequent, depending on time since treatment and individual circumstances. Follow-up included a subjective physical examination with assessment of motor function and routine surveillance imaging as directed by the managing oncologists was performed, typically by a radiation oncologist or neurosurgeon with expertise in skull-base malignancies.
To identify and grade brainstem toxicity, we collected information from patient-reported assessments or medical records. Patient-reported assessments were completed either through an online questionnaire completed by the patient or by phone interview with a member of the radiation oncology care team, usually a physician. The motor assessment components of the questionnaire evaluated muscle weakness and neurologic dysfunction including interference with activities of daily living. Regarding physical examinations by the following physician, we accepted documented evaluation of motor function and continued radiographic follow-up. If office notes with the physical examination or documentation of recent imaging were unavailable, we asked the following physician to complete a standard follow-up form including death status, local control status, and development of ‘any significant late effects (grade 3 and 4 toxicity according to CTCAE criteria)’. Oncologists who specialize in the care of skull-base tumors completed these updates and documented the date of last follow-up examination. These oncologists are aware of the risk of radionecrosis and routinely perform basic assessments of these patients in clinic. All events of brainstem necrosis were evaluated within a multidisciplinary neuroradiology conference. Toxicity is reported using the cumulative incidence method and details of other central nervous system (CNS) have been previously reported [Citation12,Citation13,Citation15].
Results
Patient characteristics
In total, 163 patients received a minimum of 45 GyRBE to 0.03 cm3 of the brainstem. Patient and tumor characteristics are summarized in . The median patient age was 50 years (range 23–81 years). Over half of the patients had at least one baseline medical condition with a known potential contributor to central nervous system injury, most commonly hypertension (37%) and smoking (26%). Several patients had multiple comorbidities. More patients were treated for conventional chordoma (63%) than chondrosarcoma (37%), and most cases represented a primary (91%) rather than recurrent disease following initial surgery alone (9%) on presentation. No patients with mesenchymal chondrosarcoma were included.
Table 1. Patient, tumor, and treatment characteristics (N = 163).
Treatment characteristics
All patients had a histologic diagnosis and had undergone either biopsy or subtotal resection (n = 137) or gross total resection (n = 26) followed by radiotherapy. Treatment characteristics, including surgical and radiotherapy details, are listed in . Most patients were treated with double-scattered (DS) proton therapy, although a few patients were treated entirely with or with a component of pencil-beam scanning (PBS) proton therapy (n = 18, 11%) or a combination of protons and photons (n = 7, 4%). A few patients (n = 4, 2%) received one to five fractions of photon therapy due to proton cyclotron downtime. No patients received chemotherapy concurrent with radiotherapy, and the only patients who were reported to receive systemic therapy were administered imatinib at the time of disease recurrence.
Outcomes
With a median follow-up of 3.9 years (range 7 months–13.8 years), the 5-year actuarial rate of brainstem toxicity was 1.3% (95% CI 0.25–4.3%), as shown in . The median follow-up of patients living without disease (n = 120) was 4.8 years (range 1.1 years–13.8 years). As noted in Supplemental Table 1, only 2 cases of brainstem toxicity occurred. Ten months following treatment, Patient A demonstrated symptoms of pyramidal weakness and medullary T2-weighted signal intensity and enhancement on imaging. Clinical and radiographic symptoms promptly resolved with steroids and thus the toxicity was categorized as grade 2. Patient B, who had preexisting hypertension, diabetes, and anoxic brain injury at birth, developed symptomatic brainstem radionecrosis 21 months after completing radiotherapy. This patient’s symptoms improved with steroids but the patient was switched to bevacizumab secondary to steroid intolerability, and the complication was graded as 3 given multiple medical interventions. Both patients also had synchronous non-brainstem CNS events. The first patient developed grade 3 temporal radionecrosis and the second was suspected of developing radiation-induced optic neuropathy with complete unilateral vision loss, both occurring after their brainstem toxicities.
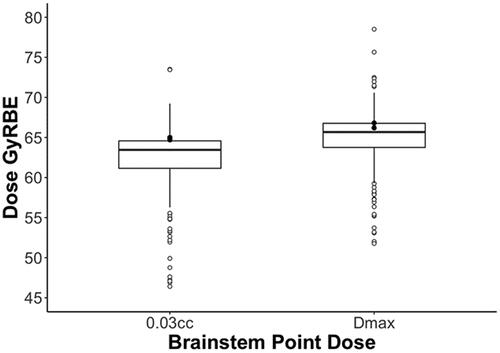
Dosimetrically, the median (and lower and upper quartile) point doses to the brainstem were as follows: 2 cm3 was 52.5 (46.9–56.1) GyRBE, 0.1 cm3 was 62.4 (59.7–63.6) GyRBE, 0.03 cm3 was 63.5 (61.2–64.6) GyRBE, and Dmax was 65.7 (63.8–66.8) GyRBE. The point dose summary statistics are illustrated in , and the 2 isolated events are represented for visual comparison. The median (and quartile) point doses for 0.03 cm3 and Dmax to the brainstem core were 56.2 (52.2−58.2) GyRBE and 59 (55.5−60.6) GyRBE, respectively. The median (and quartile) doses by volume were as follows: V50 was 3 (1.4−5.4) cm3, V54 was 1.6 (0.8−3) cm3, and V60 was 0.3 (0.9−0.7) cm3. The median (and quartile) brainstem D10%, D50%, and D90% doses were 51.1 (44.4−54.4) GyRBE, 28 (15.1−35.5) GyRBE, and 4.6 (0.6−11.8) GyRBE, respectively.
Discussion
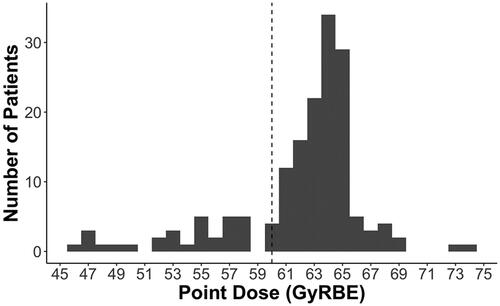
Dose escalation above 70 Gy, treatment at high-volume centers, and the use of proton therapy for individuals with chordoma and chondrosarcoma have been associated with prolonged survival [Citation3,Citation4]. Given the proximity of these tumors to sensitive OARs, compromises in target coverage or dose constraints are commonplace, even with highly conformal radiotherapy delivery techniques. The present series demonstrates that the incidence of grade 2 or higher brainstem radionecrosis was very low when delivering highly conformal dose-escalated RT without chemotherapy, and provides a rare opportunity to help define the upper bounds of the brainstem dose threshold. As noted in illustrating the point dose (D0.03 cc) frequencies, over three quarters of the patients within this series were treated with doses above those currently being used on cooperative group studies for skull-based malignancies, and almost 40% received over 64 GyRBE, which is the upper limit of the qualitative analyses of normal tissue effects in the clinic (QUANTEC) guidelines [Citation5,Citation8,Citation9,Citation11]. Furthermore, no patients experienced brainstem events that did not respond to medical management.
Figure 4. Histogram of the brainstem point dose constraint to 0.03 cm3. Overall, 131 and 64 individuals received 60 and 64 Gy or more, respectively. The vertical dotted horizontal line reflects the patients who received more than 60 Gy, which is a common constraint in many cooperative group protocols.
While the present study reports a high dose threshold for adults, this contrasts with the rate of toxicity observed within the pediatric populations, which occurs at lower dose limits. Indelicato et al. reviewed the incidence of brainstem complications among contemporaneous pediatric patients treated at the University of Florida [Citation16]. They noted that while the rate of grade 3 or higher injury was 2.1%, those below age 5 years had a significantly higher rate of brainstem toxicity (6.9 vs. 1.1%, p < 0.01). Several factors are thought to increase pediatric patients’ sensitivity to injury, including still-developing neural structures, predominantly intra-axial posterior fossa tumor locations, and certain chemotherapy practice patterns. A more recent study providing an overview of the NCI workshop on proton therapy for children reviewed the evidence contributed by the 3 largest pediatric proton therapy centers in the United States, including the University of Florida. This manuscript summarizes the results for 671 children with focal posterior fossa tumors. While the overall incidence of brainstem toxicity was 2.4%, by enacting more stringent protocols, including limiting D0.1 cc to less than 58 Gy, the rate may be as low as 0% [Citation17]. As the present series focuses on largely extra-axial, skull-based tumors in patients who predominantly underwent anterior-based endoscopic surgeries and did not receive adjuvant systemic therapies, caution should be taken when comparing to other datasets.
While children are predisposed to brainstem complications at lower dose thresholds, other adult series corroborate the low rates of brainstem radionecrosis with dose-escalated radiotherapy. Debus et al. evaluated 367 patients treated with photons and protons. They identified that Dmax >64 GyRBE, V50 > 5.9 cm3, V55 > 2.7 cm3, V60 > 0.9 cm3, 2 or more skull-based surgeries, diabetes, and hypertension were associated with a higher incidence of brainstem necrosis. Although within the present cohort over 70% of the patients were treated to a point dose (Dmax) above 64 GyRBE to the brainstem, the median to V50, V54, and V60, were generally low in which the upper quartile for V50, V54, and V60 observed was at 5.4 cm3, 3 cm3, and 0.7 cm3, respectively. The findings indicate that a high dose to the surface of the brainstem is safe if volumetric thresholds to the center of the brainstem can be achieved.
Further supporting our experience, investigators at Paul Scherer Institute published their 2016 series reviewing 222 patients with skull-base chordoma and chondrosarcoma treated to a mean dose of 72.5 GyRBE with pencil-beam therapy. They noted that the 7-year toxicity-free survival rate was 87.2%. Although 32% of patients had brainstem compression, their group did not observe a single brainstem toxicity [Citation10]. Feuvret et al. and Fung et al. published among the largest proton outcomes series of skull-base chondrosarcoma and chordoma from a French consortium. With a vast number of patients across both datasets having tumor near the brainstem receiving 64 GyRBE up to 1.5 cm3, they reported no brainstem injuries across both series [Citation18,Citation19]. Lastly, our findings compare similarly to results found in photon cohorts treated with highly conformal image-guided techniques. Sahgal et al. reported on 24 chordoma and 18 chondrosarcoma patients treated to up to 76 Gy with intensity-modulated radiotherapy with a median brainstem Dmax and range of 67.65 Gy (4.60–73.50) and 64.85 Gy (36.60–78.90) for treatment of chordoma and chondrosarcoma, respectively [Citation20]. These datasets reaffirm the upper limits of brainstem tolerance for maximum point dose to the surface.
The RBE of protons relative to photons has historically been estimated to be constant at 1.1; however, there is laboratory evidence that the RBE dose is higher at the distal end of a spread-out Bragg peak, and may pose greater risk to critical CNS OARs distal to the beam path [Citation21,Citation22]. These concerns have been highlighted in a recent collaboration with investigators from Massachusetts General Hospital who calculated the RBE for temporal lobe enhancement from proton treatments to be 1.18, and the dose tolerance at D1% to be 58.56 GyRBE for protons and 69.07 Gy for photons [Citation23]. Eighty-five percent of the patients within the present series had tumor within 5 mm of the brainstem, and all received a minimum 45 GyRBE to the point max (D0.03), removing any outliers with no potential clinical risk of brainstem toxicity. Regardless of theoretical differences in end-range RBE, there is no evidence that dose escalation as currently employed by high-volume proton centers confers a greater risk of injury to patients treated with photons.
Based on the study design and the few events, our statistical analysis of factors associated with brainstem toxicity was limited, which is expected, given the rarity of the event. Additionally, as with many rare disease entities treated at international referral centers, the digital imaging and communications in medicine (DICOM) for the entire dataset was not available for independent, central review from all routine follow-up examinations. This was directed by the local team, who typically included oncologists who specialize in the treatment of skull-base malignancies. As such, we excluded grade 1 toxicities from our analysis since the rate of clinically asymptomatic events is more likely to be underreported. Noted in Supplemental Table 1, both patients with toxicities had other suspected CNS events, suggesting that brainstem complications are multifactorial and there may be other biologic co-variates beyond dose alone that predict for toxicity. Further research is needed to assess which individualized treatment parameters can best predict its occurrence and the results of the present study may not be generalizable to other diseases and patient populations.
When using highly conformal techniques, treating to a point dose (D0.03) of the brainstem surface up to of 64 GyRBE without chemotherapy poses minimal risk of irreversible brainstem toxicity in adult patients. Based on our current treatment protocols and practice, we recommend using a brainstem constraint of D0.1 cm3 <64 GyRBE to the surface of the brainstem and D0.1 cc <60 GyRBE to the brainstem core.
Supplemental Material
Download MS Word (17.8 KB)Acknowledgement
The authors report no funding.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors agree to share anonymized data upon reasonable request by researchers.
Notes
* Abstract presented at the annual Particle Therapy Co-Operative Group (PTCOG) meeting in Miami, FL on June 30th, 2022.
References
- Mercado CE, Holtzman AL, Rotondo R, et al. Proton therapy for skull base tumors: a review of clinical outcomes for chordomas and chondrosarcomas. Head Neck. 2019;41(2):536–541.
- Lawrence YR, Li XA, el Naqa I, et al. Radiation dose-volume effects in the brain. Int J Radiat Oncol Biol Phys. 2010;76(3):S20–S7.
- Palm RF, Oliver DE, Yang GQ, et al. The role of dose escalation and proton therapy in perioperative or definitive treatment of chondrosarcoma and chordoma: an analysis of the national cancer data base. Cancer. 2019;125(4):642–651.
- Holtzman AL, Bates JE, Morris CG, et al. Impact of type of treatment center and access to care on mortality and survival for skull base chordoma and chondrosarcoma. J Neurol Surg Part B Skull Base. 2021;83(3):328–338.
- Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991; 21(1):109–122.
- Debus J, Hug EB, Liebsch NJ, et al. Brainstem tolerance to conformal radiotherapy of skull base tumors. Int J Radiat Oncol Biol Phys. 1997;39(5):967–975.
- Debus J, Hug EB, Liebsch NJ, et al. Dose-volume tolerance of the brainstem after high-dose radiotherapy. Front Radiat Ther Oncol. 1999;33:305–314.
- Rogers CL, Won M, Vogelbaum MA, et al. High-risk meningioma: Initial outcomes from NRG oncology/RTOG 0539. Int J Radiat Oncol Biol Phys. 2020;106(4):790–799.
- Rogers L, Zhang P, Vogelbaum MA, et al. Intermediate-risk meningioma: initial outcomes from NRG oncology RTOG 0539. J Neurosurg. 2018;129(1):35–47.
- Weber DC, Malyapa R, Albertini F, et al. Long term outcomes of patients with skull-base low-grade chondrosarcoma and chordoma patients treated with pencil beam scanning proton therapy. Radiother Oncol. 2016;120(1):169–174.
- Mayo C, Yorke E, Merchant TE. Radiation associated brainstem injury. Int J Radiat Oncol Biol Phys. 2010; 76(3):S36–S41.
- Holtzman AL, Rotondo RL, Rutenberg MS, et al. Clinical outcomes following dose-escalated proton therapy for skull-base chordoma. Int J Part Ther. 2021;8(1):179–188.
- Holtzman AL, Rotondo RL, Rutenberg MS, et al. Proton therapy for skull-base chondrosarcoma, a single-institution outcomes study. J Neurooncol. 2019; 142(3):557–563.
- Deraniyagala RL, Yeung D, Mendenhall WM, et al. Proton therapy for skull base chordomas: an outcome study from the university of Florida proton therapy institute. J Neurol Surg B Skull Base. 2014;75(1):53–57.
- De Leo AN, Holtzman AL, Ho MW, et al. Vision loss following high-dose proton-based radiotherapy for skull-base chordoma and chondrosarcoma. Radiother Oncol. 2021;158:125–130.
- Indelicato DJ, Flampouri S, Rotondo RL, et al. Incidence and dosimetric parameters of pediatric brainstem toxicity following proton therapy. Acta Oncol. 2014;53(10):1298–1304.
- Haas-Kogan D, Indelicato D, Paganetti H, et al. National cancer institute workshop on proton therapy for children: considerations regarding brainstem injury. Int J Radiat Oncol Biol Phys. 2018;101(1):152–168.
- Fung V, Calugaru V, Bolle S, et al. Proton beam therapy for skull base chordomas in 106 patients: a dose adaptive radiation protocol. Radiother Oncol. 2018;128(2):198–202.
- Feuvret L, Bracci S, Calugaru V, et al. Efficacy and safety of adjuvant proton therapy combined with surgery for chondrosarcoma of the skull base: a retrospective, population-based study. Int J Radiat Oncol Biol Phys. 2016;95(1):312–321.
- Sahgal A, Chan MW, Atenafu EG, et al. Image-guided, intensity-modulated radiation therapy (IG-IMRT) for skull base chordoma and chondrosarcoma: preliminary outcomes. Neuro Oncol. 2015;17(6):889–894.
- Sorensen BS, Bassler N, Nielsen S, et al. Relative biological effectiveness (RBE) and distal edge effects of proton radiation on early damage in vivo. Acta Oncol. 2017;56(11):1387–1391.
- Paganetti H, Blakely E, Carabe-Fernandez A, et al. Report of the AAPM TG-256 on the relative biological effectiveness of proton beams in radiation therapy. Med Phys. 2019;46(3):e53–e78.
- Zhang YY, Huo WL, Goldberg SI, et al. Brain-Specific relative biological effectiveness of protons based on long-term outcome of patients with nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2021;110(4):984–992.