Abstract
Background
The benefits of exercise training are well documented among breast cancer (BC) survivors. Patients decrease their physical activity during treatment, and many fail to regain their previous exercise levels. There is therefore a need to define factors supporting long-term physical activity behavior in this patient group, to target supporting interventions aimed at preventing the decline in physical activity (PA).
Aim
The aim of this study was to determine physical and psychosocial factors explaining long-term physical activity after the adjuvant treatments in BC survivors.
Methods
Four-hundred forty-six BC survivors followed for 5-years within a randomized exercise trial participated. Factors explaining (1) physical activity after the adjuvant treatments and (2) changes in physical activity in long-term were analyzed using linear regression models and general estimating equation models. Pretreatment leisure-time physical activity (LTPA), demographic, and treatment factors, physical fitness, and quality of life (Qol) at baseline were independent factors.
Results
Exercise levels increased during the first year, and thereafter remained mostly stable. Higher LTPA, higher fitness level, better Qol and older age at baseline were associated with higher physical activity level after adjuvant treatments (p < .001) in multivariate analysis. Higher levels of fatigue (p < .008) and better emotional functioning (p = .017) at baseline were the main factors associated with increased physical activity during the follow-up.
Conclusion
Previous exercise habits and Qol after adjuvant chemo-, and radiotherapy were the strongest determinants of long-term physical activity levels in breast cancer survivors. Patients with better emotional functioning increased their exercise activity most as did those patients with higher fatigue levels at baseline. Patients suffering from fatigue after adjuvant treatment managed to increase their exercise levels, in contrast to patients with low emotional functioning, and may benefit from physical exercise interventions. Emotionally deprived patients may benefit from psychosocial support to regain their previous exercise levels.
Introduction
The prognosis of breast cancer (BC) has improved steadily during several decades due to advances in diagnostics as well as improved adjuvant treatments. Presently, the European 5-year survival of BC is over 80% and 91% in Finland (www.cancerregistery.fi). Previous studies have shown, that breast cancer patients’ health-related quality of life (HRQol) often decreases during adjuvant treatments [Citation1,Citation2]. Effective, but burdensome adjuvant treatments, can also cause long-term adverse effects, such as early menopause, declined bone mineral density, increased risk of cardiovascular diseases (CVD) and fatigue, which may contribute to the reduction of general QoL.
Exercise may prevent osteoporosis [Citation3–5], help in weight control, and reduce the risk for CVD and depression among cancer survivors [Citation6–9]. Exercise interventions have shown to improve patients’ self-confidence and to lower their threshold to enhance physical activity level after cancer [Citation10–15]. Exercise improves self-reported physical activity [Citation16–18], reduces pain and fatigue disorders [Citation18–21] and increases the patients’ overall HRQol [Citation2,Citation18,Citation20,Citation22–24]. High physical activity both before and after cancer diagnosis is associated with a lower risk of cancer recurrence and all-cause mortality [Citation25–29].
There is an urgent need for more holistic approaches and interdisciplinary treatments to improve the HRQol among the rapidly increasing number of BC survivors [Citation25,Citation30,Citation31]. Despite the willingness of cancer survivors to change their lifestyle [Citation10,Citation14,Citation32], physical activity is known to decrease after the cancer diagnosis due to treatments [Citation9,Citation11,Citation20,Citation33]. Many cancer survivors do not fulfill the general physical activity recommendations [Citation11,Citation24]. Factors motivating cancer survivors to retain their physical activity behavior have been studied in single studies [Citation34–42], overviews and meta-analyses [Citation24,Citation40,Citation43,Citation44]. Yet many studies have been small, and the number of prospective studies conducted breast cancer patients after adjuvant treatment is still limited [Citation34–39]. Multiple physical, psychological and psychosocial factors have been perceived limiting physical activity after cancer, such as previous exercise history, self-efficacy, fatigue, kinesiophobia and low motivation [Citation24,Citation44]. Only a few larger, prospective studies of BC patients with systematic longitudinal assessment of physical activity after adjuvant treatment have been published [Citation34–36]. Thus, there is still a need to clarify factors affecting long-term physical activity.
BREX (BReast cancer and EXercise) study is one of the largest exercise intervention trials in breast cancer survivors. The exercise intervention prevented femoral neck bone loss in premenopausal patients [Citation45–47]. There were no differences in QoL or exercise habits between the two randomized groups in follow-up [Citation18,Citation21]. The aim of the present study was to investigate factors associated with physical activity after adjuvant treatments and during 5 years of follow-up.
Methods
Patients
The BREX study was a national, multicentered, randomized controlled exercise trial conducted in Helsinki, Tampere, and Turku University Hospitals between 2005 and 2017. Pre- and postmenopausal women aged 35–68 years with newly diagnosed BC and with recently completed adjuvant chemotherapy or at start of endocrine therapy could enter the study [Citation48]. Adjuvant therapy was given according to national clinical guidelines. The patients were randomized into either a 12-month physical exercise intervention or control after adjuvant chemotherapy and radiotherapy and were followed with regular clinical, laboratory investigations as well as testing their life-style factors, exercise habits, physical fitness and HRQoL. The primary objectives of original BREX were to study the effect of exercise on bone health and HRQoL [Citation48]. The results have been previously reported elsewhere [Citation6,Citation20,Citation21,Citation23,Citation48].
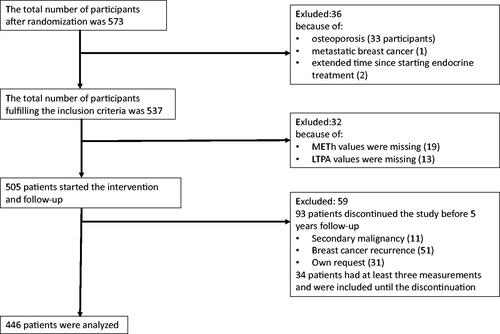
The total number of participants filling the study inclusion criteria was 537. LTPA information was missing from 13 patients. They were excluded from the present study. Baseline metabolic equivalent per hour (METh) values were missing from 33 patients. Baseline METh-values were imputated for 14 of these but data was insufficient for imputation in 19 patients, who were excluded. During the 5-year follow-up, 93 patients discontinued the study (11 because of new malignancy, 51 had BC recurrence and 31 discontinued on their own request). These 93 patients were included until discontinuation. However, 59 of them had less than three METh measures before termination of the study and were excluded. Thus, 446 patients were included in the final analyses. A flow chart of patient inclusion is shown in .
The local Ethical Committee of the Helsinki University Hospital approved the study protocol. Informed consent was obtained from all individual participants before entering the study. The trial was registered in the Helsinki and Uusimaa Hospital District Clinical Trials Register (www.hus.fi; trial number 210590) and in the clinical trial website (http://www.clinicaltrials.gov/; identifer number NCT00639210).
As we have previously reported, there were no significant differences in physical activity (METh) between the exercise and control groups during the 5-year follow-up [Citation18]. Therefore, we combined the exercise and control groups in the present study. The main results of the primary BREX intervention are represented more detailed elsewhere [Citation18,Citation20,Citation21,Citation23,Citation48].
Exercise intervention
After the baseline visit, the patients were randomized either into 12-month supervised exercise training group or control group. The exercise intervention consisted of both supervised and home training protocol. The supervised training was organized once a week as a 60-min endurance program and rotating between step-aerobics and a circuit-training with switch every fortnight. The intensity of exercise was assessed by a Rating of Perceived Exertion (RPE) scale, which relies on self-estimation of stress level. After the first six weeks of less intensive training, the stress level was raised toward 14–16 RPE’s [Citation49]. This equals to exercise that feels ‘somewhat hard’ or ‘hard’ and corresponds to 5–7 metabolic equivalents (METs). A metabolic equivalent (MET) unit is the amount of oxygen consumed at rest in supine position and matches 3.5 ml oxygen consumption per kilogram each minute [Citation50]. The home training sessions included endurance training twice-a-week. The nonsupervised endurance training consisted of walking, Nordic walking, or aerobic training. The control group was recommended to maintain their usual level of physical activity and exercise habits during the follow-up.
The medical history was recorded at baseline visit after the adjuvant treatments. The patients filled questionnaires for Qol, basic demographics, lifestyle issues, work-life, and leisure-time exercise history. The questionnaires were repeated every six months, up to 36 months and then at 5-year follow-up. Self-reported leisure-time physical activity (LTPA) before the diagnosis was assessed by a questionnaire. Three levels of physical activity were determined by the amount and intensity defined: I Low intensity (e.g., watching tv or reading), II moderate intensity (e.g., walking, slow cycling at least 4 h per week) and III vigorous intensity (e.g., swimming, ball games, jogging or gym at least 3 h per week). The physical activity intensity was assessed with a prospective, two-week exercising diary and during the follow-up every six-months until 36-months and then again at the cut-point of 60-months (5-years). In this diary, the physical activity intensity was categorized from light to very vigorous on a metabolic equivalence (MET) scale using intensity categories of 3, 3–6, 6–9 and >9 METh/week [Citation51]. The physical fitness was tested at baseline and after the intervention, then at three and five-year follow-ups by a 2-km walk test (UKK walk test, Tampere, Finland) [Citation52].
The QoL and symptoms were measured with the EORTC QLQ-C30 [Citation53] and the BC specific BR-23 [Citation54] questionnaires. The questionnaires were filled at the baseline, and then after 6, 12, 24, 30, 36 and 60-months respectively.
Statistical analysis
The descriptive statistics were presented as means with SD’s or as counts with percentages. Statistical comparisons between groups were done using analysis of variance (ANOVA), and a chi-square test. Variables characterizing demographics, tumor characteristics, treatment, QoL, physical fitness and activity were studied as predictive factors.
Univariate and multivariate regression models with forward stepwise selection (probability for entry ≤0.05, probability for removal ≥0.10) were used to investigate factors related to physical activity (METh) with standardized regression coefficient Beta (β). The Beta value is a measure of how strongly the predictor variable influences the criterion variable. The Beta is measured in units of SD. Cohen’s standard for Beta values above 0.10, 0.30 and 0.50 represents small, moderate, and large relationships, respectively. Repeated measures were analyzed using generalizing estimating equations (GEE) models with unstructured correlation structure. Fixed effects were group, time, and group-time interactions. Generalized estimating equations were developed as an extension of the general linear model (e.g., OLS regression analysis) to analyze longitudinal and other correlated data. GEE models allowed the analyses of unbalanced datasets without imputation; therefore, we analyzed all available data with the full analysis set. In the case of violation of the assumptions (e.g., non-normality) for continuous variables, a bootstrap-type method was used. The area under the curve (AUC) was calculated from all time points during follow-up after baseline using the trapezoidal method; the baseline value was subtracted to get the average change in time (AUC minus baseline). The AUC method estimates physical activity as the average METh/week during the whole 5-year follow-up. The normality of variables was evaluated graphically and by using the Shapiro–Wilk W test. Stata 17.0, StataCorp LP (College Station, TX, USA) statistical package was used for statistical analyses.
Results
The total number of patients included in this study was 446. Patient characteristics according to self-reported leisure-time physical activity (LTPA) before the BC diagnosis are shown in .
Table 1. Patient characteristics.
Factors associated with physical activity at baseline
A higher physical activity (METh/week) was significantly associated with a higher previous LTPA level before BC diagnosis. Higher global QoL, older age and a better scoring in the 2-km walk-test at baseline were associated with a higher physical activity in a multivariate analysis but not in univariate analysis. High fatigue score was significantly associated with a low physical activity in univariate, but not in a multivariate analysis. (). The most important factor for identifying patients with low physical activity at baseline, according to the strength of the standardized beta coefficient, was the previous LTPA followed by physical fitness and age.
Table 2. Univariate and multivariate linear regressions models of physical activity (METh/week) at baseline.
Physical activity during the 5-year follow-up period
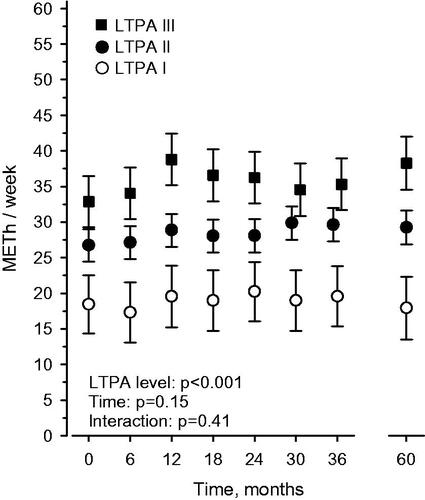
High LTPA level (LTPA III) before BC diagnosis was statistically significantly associated with higher physical activity (METh/week) during the whole 5-year follow-up period (p < .001) ().
Factors associated with change in physical activity
The factors associated with change in METh/week from baseline to the average between 6 and 60 months of follow-up are presented in . Multivariate forward stepwise analyses showed that a high emotional functioning (EF) level was significantly associated with an increase in the physical activity after baseline (p = .017) as was a lack of significant comorbidities at borderline significance (p = .05). The most important factors which predicted increase in physical activity, according to the strength of the standardized beta coefficient, were; (1) a high level of fatigue followed by (2) good emotional functioning and (3) a low score of comorbidities at baseline.
Table 3. Univariate and multivariate linear regression models for change in physical activity during 5-year follow-up (MET AUC6-60 minus baseline).
A high level of fatigue at baseline was significantly associated with increased physical activity during follow-up (p = .008). Physical activity during the whole five-year follow-up period according to baseline fatigue score tertiles is shown in Supplemental Figure 3. A high baseline fatigue score was associated with low physical activity at baseline but the differences in activity levels between the tertiles diminished during follow-up. At five year the moderate fatigue tertile group had reached the level of the lowest tertile, while the activity level of the high fatigue group remained lower throughout follow-up.
Discussion
Our study revealed that the physical activity after BC treatment was strongly associated with the level of leisure time physical activity before the BC diagnosis. Other factors affecting post-treatment exercise levels were QoL, baseline fitness level as measured with a 2-km walk-test and age. Exercise levels increased during the first year of the study, especially among those with a high pretreatment LTPA [Citation18,Citation21,Citation55]. Thereafter, during the 5-year follow-up period, the physical activity level remained mostly stable. Our results indicate that posttreatment exercise habits in BC survivors are determined by pretreatment lifestyles and routines to a high degree. This has been shown also previously in several studies, although with a limited follow-up time compared to our study [Citation12,Citation44,Citation56,Citation57]. Also in healthy individuals, learned exercising behavior has been shown to remain largely stable across the lifespan as shown in the large twin-cohort study by van der Zee et al., the [Citation56].
Cancer survivors are willing to adopt life-style changes [Citation10,Citation58,Citation59] and most of them are interested in better lifestyle and exercise counseling [Citation32,Citation60]. Cancer survivors are highly motivated for behavioral changes during the first 3–5 months after the oncologic treatment [Citation58]. Regaining physical activity after treatment is an incentive to return to a normal physical condition [Citation43]. Previously published results from the BREX study support this [Citation18,Citation20,Citation21]. Motivators, such as weight management, body image and health improvement have been identified to be drivers for the maintenance of physical activity [Citation24,Citation40,Citation43]. Although patients randomized to supervised exercise were assumed to participate in a highly demanding and time-consuming exercise intervention for a full year, 78% of eligible patients were willing to participate [Citation21,Citation48]. All participants increased their amount of exercise significantly during the intervention (the first trial year) [Citation21,Citation55] and this increase was similar in the exercise and control groups. Our interpretation was, that participation in the trial, with regular questionnaires of exercise habits as well as testing the physical fitness, encouraged and motivated patients in the control group as much as those in the supervised training group [Citation18,Citation48]. A randomized trial of Pinto et al., reported that telephone counseling to support physical activity by health-care providers at 3 and 6 months was sufficient to motivate exercising [Citation61,Citation62] which supports our interpretation. A previous qualitative investigation emphasized the need for a safe goal setting and monitoring by health professionals as guidance for exercise training [Citation41]. Thus, for many regularly exercising patients, returning to previous activity levels before the cancer, seems to be possible. For physically active patients, it seems, that simply encouraging to retain active lifestyle would be enough for gaining physical activity levels after BC [Citation44,Citation59,Citation63].
The long-term physical activity levels of most patients with low previous levels of regular physical exercise remained low in the present study. Our results support the results from previous studies including a recently updated Cochrane review (2018) and a qualitative study from Monteiro-Guerra et al. and emphasizes the challenges of achieving long-term lifestyle changes in cancer survivors [Citation11,Citation41). Recommended physical exercise levels may be sustained via interventions and by affecting the behavioral habits, but only for a limited period (follow-up from 3–6 months) [Citation11]. This might at least partly explain the lack of long-lasting improvement in physical activity levels in most patients with low levels of previous LTPA. Future research should focus on individual support and physical education including instructed rehabilitation guidance targeted for this group of patients.
Although the physical activity remained mostly stable during the 5-year follow-up, a proportion of participants managed to increase their exercise levels. Patients with impaired emotional functioning and comorbidities seem to lack the necessary resources for changing their exercise habits after treatment [Citation24]. Such patients are easily excluded from exercise trials, and may not be motivated for volunteering to participate in physical exercise studies [Citation24]. Not unexpectedly, patients with comorbidities also had a reduced capacity to increase their exercise level. On the other hand, patients suffering from fatigue at baseline, while having low physical activity levels at outset, managed to improve their exercise levels. Baseline fatigue level was the factor most significantly associated with increased exercise levels during follow-up. In the present study, most patients reported some level of fatigue and 40% of them moderate or severe fatigue (data not shown). The association of high levels of baseline fatigue to improved physical activity during the trial follow-up may seem counterintuitive at first. This may naturally be due to chance in this analysis including many potential explaining factors. The phenomenon should therefore be verified in an independent study. On the other hand, this finding may imply that fatigue after treatments is not a permanent obstacle for overcoming treatment-related decline in physical activity.
Emotional well-being and vitality as an important part of good QoL are needed for lifestyle transitions, since the emotional symptom burden decreases motivation and initiative [Citation12,Citation56,Citation64]. Kampschoff et al. stressed the importance of psychological factors, such as self-efficacy and distress for adherence to vigorous exercise training interventions [Citation44,Citation65,Citation66], in line with our findings.
Fatigue is a more complex phenomenon. Fatigue is defined as a distressing, persistent, subjective sense of physical, emotional, and/or cognitive tiredness, which is not proportional to recent physical activity and interferes with usual functioning [Citation67]. Previous studies with our patient cohort have revealed significant associations between physical inactivity, depression, fatigue and impaired QoL [Citation20,Citation21]. A recent GLASSO network analysis describing a significant interplay between depression, symptoms, and level of physical functioning during the first year of the BREX study revealed that two symptom clusters, were most closely associated with lower QoL; fatigue and impaired physical function on one hand and depression and decreased emotional function on the other hand [Citation68]. Thus, in the present patient cohort it seemed that fatigue was more related to physical functioning than to depression. Fatigue reduces physical activity and further impairs functional capacity and QoL [Citation21,Citation69]. Fatigue restricts the adherence for exercise programs [Citation24]. Yet, exercising reduces fatigue, also after cancer treatments [Citation21,Citation63,Citation70].
Patients with high emotional distress and lower total Qol are at risk for discontinuing their exercise activities. These survivors could benefit from targeted, psychosocial emphasized interventions integrated as a part of individual rehabilitation programs in the early follow-up phase after BC while motivation for behavioral changes may be highest [Citation58], followed by encouraging counseling by healthcare professionals, as shown in previous investigations [Citation41,Citation61,Citation62]. Patients with high levels of fatigue might benefit from more simple interventions like encouraging physically active lifestyle or individual physical rehabilitation counseling.
The main strengths of this study were the large, homogenous study population, the length of the intervention (12 months) and follow-up (5-years). To our knowledge, this is the largest exercise study in any cancer population, with the longest follow-up time. Moreover, compliance was high as the participant rate remained over 80% after five-years of follow-up. The intervention was well-monitored with regular assessment of physical activity (METh/week). The main limitation of this study, as of exercise intervention studies in general, is the selection of mainly physically active persons, which contributes to a ceiling effect in the ability to improve physical activity during the intervention and thereafter. Secondly, patients with major musculoskeletal comorbidities were excluded, since the exercise intervention required good mobility. A third limitation is the lack of prospectively collected data of physical exercise and quality of life before and during anticancer treatments. As always, randomized intervention studies without blinding facilitates also controls, like in the present trial. It did not utilize qualitative study methodology.
Conclusions
Our study demonstrated the importance of previous exercise habits for retaining long-term physical activity after adjuvant treatments in BC survivors. The individual level of physical activity remains mostly constant after exercise intervention signifying the exercise habits adopted earlier in life. We found the impaired emotional well-being as part of general Qol being an important obstacle for transition in exercising habits. A high baseline fatigue level was the factor most strongly associated with increased PA during follow-up. These findings might help to identify the patients in need of early support and individual rehabilitation planning during the survivorship-care to restore their Qol, physical fitness and further functional capacity.
| Abbreviations | ||
| BC | = | Breast cancer |
| BREX | = | Breast cancer and exercise trial |
| QoL | = | quality of life |
| METh | = | metabolic equivalent per hour |
| PE | = | Physical exercise |
| PF | = | physical fitness |
| PA | = | Physical activity |
| LTPA | = | Leisure-time physical activity |
Supplemental Material
Download MS Word (18.1 KB)Acknowledgements
The authors thank the whole BREX research group, the volunteers and the patients who participated in the primary intervention path and made it possible. The authors had the permission of the EORTC-QLQ-C30 for using their original questionnaire. The authors also thank the Finnish Breast Cancer Group for sponsoring the primary investigation.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Roine E, Sintonen H, Kellokumpu-Lehtinen PL, et al. Health-related quality of life of breast cancer survivors attending an exercise intervention study: a five-year follow-up. In Vivo. 2020;34(2):667–674.
- Roine E, Sintonen H, Kellokumpu-Lehtinen PL, et al. Long-term health-related quality of life of breast cancer survivors remains impaired compared to the age-matched general population especially in young women. Results from the prospective controlled BREX exercise study. Breast. 2021;59(Journal Article):110–116.
- Niu K, Ahola R, Guo H, et al. Effect of office-based brief high-impact exercise on bone mineral density in healthy premenopausal women: the sendai bone health concept study. J Bone Miner Metab. 2010;28(5):568–577.
- Dalla Via J, Daly RM, Fraser SF. The effect of exercise on bone mineral density in adult cancer survivors: a systematic review and Meta-analysis. Osteoporos Int. 2018;29(2):287–303.
- Vainionpaa A, Korpelainen R, Vaananen HK, et al. Effect of impact exercise on bone metabolism. Osteoporos Int. 2009;20(10):1725–1733.
- Elme A, Utriainen M, Kellokumpu-Lehtinen P, et al. Obesity and physical inactivity are related to impaired physical health of breast cancer survivors. Anticancer Res. 2013;33(4):1595–1602.
- Sturgeon KM, Ky B, Libonati JR, et al. The effects of exercise on cardiovascular outcomes before, during, and after treatment for breast cancer. Breast Cancer Res Treat. 2014;143(2):219–226.
- Kruk J. Physical activity in the prevention of the most frequent chronic diseases: an analysis of the recent evidence. Asian Pac J Cancer Prev. 2007;8(3):325–338.
- Irwin ML, Ainsworth BE. Physical activity interventions following cancer diagnosis: methodologic challenges to delivery and assessment. Cancer Invest. 2004;22(1):30–50.
- Livsey L, Lewis K. Breast cancer survivors’ perceptions of participating in a supervised exercise intervention: An exploratory review of the literature. Women Health. 2018;58(9):1017–1036.
- Turner RR, Steed L, Quirk H, et al. Interventions for promoting habitual exercise in people living with and beyond cancer. Cochrane Database Syst Rev. 2018;9(Journal Article):CD010192.
- Craike MJ, Gaskin CJ, Mohebbi M, et al. Mechanisms of physical activity behavior change for prostate cancer survivors: a cluster randomized controlled trial. AnnBehavMed. 2018;52(9):798–808.
- Kampshoff CS, Stacey F, Short CE, et al. Demographic, clinical, psychosocial, and environmental correlates of objectively assessed physical activity among breast cancer survivors. Support Care Cancer. 2016;24(8):3333–3342.
- Luoma ML, Hakamies-Blomqvist L, Blomqvist C, et al. Experiences of breast cancer survivors participating in a tailored exercise intervention -a qualitative study. Anticancer Res. 2014;34(3):1193–1199.
- Swartz MC, Lewis ZH, Lyons EJ, et al. Effect of home- and community-based physical activity interventions on physical function among cancer survivors: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2017;98(8):1652–1665.
- Lahart IM, Metsios GS, Nevill AM, et al. Physical activity for women with breast cancer after adjuvant therapy. Cochrane Database SystRev. 2018;1(1):CD011292.
- Kampshoff CS, Chinapaw MJ, Brug J, et al. Randomized controlled trial of the effects of high intensity and low-to-moderate intensity exercise on physical fitness and fatigue in cancer survivors: results of the resistance and endurance exercise After ChemoTherapy (REACT) study. BMC Med. 2015;13(Journal Article):275.
- Penttinen H, Utriainen M, Kellokumpu-Lehtinen P, et al. Effectiveness of a 12-month exercise intervention on physical activity and quality of life of breast cancer survivors; five-year results of the BREX-study. In Vivo. 2019;33(3):881–888.
- Olsson Moller U, Beck I, Ryden L, et al. A comprehensive approach to rehabilitation interventions following breast cancer treatment – a systematic review of systematic reviews. BMC Cancer. 2019;19(1):472.
- Penttinen H, Rautalin M, Roine R, et al. Quality of life of recently treated patients with breast cancer. Anticancer Res. 2014;34(3):1201–1206.
- Penttinen HM, ST Kellokumpu Lehtinen P, Blomqvist C, et al. Hakamies blomqvist L. Quality of life and physical performance and activity of breast cancer patients after adjuvant treatments. Psycho-oncology. 2011;20(11):1211–1220.
- Wirtz P, Baumann FT. Physical activity, exercise and breast Cancer - What Is the evidence for rehabilitation, aftercare, and survival? A review. Breast Care (Basel). 2018;13(2):93–101.
- Saarto T, Sievanen H, Kellokumpu-Lehtinen P, et al. Effect of supervised and home exercise training on bone mineral density among breast cancer patients. A 12-month randomised controlled trial. Osteoporos Int. 2012;23(5):1601–1612.
- Elshahat S, Treanor C, Donnelly M. Factors influencing physical activity participation among people living with or beyond cancer: a systematic scoping review. Int J Behav Nutr Phys Act. 2021;18(1):50.
- Ibrahim EM, Al-Homaidh A. Physical activity and survival after breast cancer diagnosis: Meta-analysis of published studies. Med Oncol. 2011;28(3):753–765.
- Patel AV, Friedenreich CM, Moore SC, et al. American college of sports medicine roundtable report on physical activity, sedentary behavior, and cancer prevention and control. Med Sci Sports Exerc. 2019;51(11):2391–2402.
- Friedenreich CM. Physical activity and breast cancer: review of the epidemiologic evidence and biologic mechanisms. Recent Results Cancer Res. 2011;188(Journal Article):125–139.
- Swain CTV, Nguyen NH, Eagles T, et al. Postdiagnosis sedentary behavior and health outcomes in cancer survivors: a systematic review and meta-analysis. Cancer. 2020;126(4):861–869.
- Spei ME, Samoli E, Bravi F, et al. Physical activity in breast cancer survivors: a systematic review and Meta-analysis on overall and breast cancer survival. Breast. 2019;44(Journal Article):144–152.
- Davis MP, Temel JS, Balboni T, et al. A review of the trials which examine early integration of outpatient and home palliative care for patients with serious illnesses. Ann Palliat Med. 2015;4(3):99–121.
- Temel JS, Greer JA, El-Jawahri A, et al. Effects of early integrated palliative care in patients With lung and GI cancer: a randomized clinical trial. J Clin Oncol. 2017;35(8):834–841.
- Sturgeon KM, Fisher C, McShea G, et al. Patient preference and timing for exercise in breast cancer care. Support Care Cancer. 2018;26(2):507–514.
- Hawkes AL, Lynch BM, Youlden DR, et al. Health behaviors of Australian colorectal cancer survivors, compared with noncancer population controls. Support Care Cancer. 2008;16(10):1097–1104.
- Barbosa A, Costa AR, Fontes F, et al. Changes in health behaviours and body mass index after a breast cancer diagnosis: results from a prospective cohort study. Eur J Cancer Prev. 2019;28(4):330–337.
- Emery CF, Yang HC, Frierson GM, et al. Determinants of physical activity among women treated for breast cancer in a 5-year longitudinal follow-up investigation. Psycho-Oncology. 1. huhtikuuta. 2009;18(4):377–386.
- Hsu HT, Dodd MJ, Guo SE, et al. Predictors of exercise frequency in breast cancer survivors in Taiwan. J Clin Nurs. 2011;20(13-14):1923–1935.
- Rogers LQ, Courneya KS, Anton PM, et al. Social cognitive constructs did not mediate the BEAT cancer intervention effects on objective physical activity behavior based on multivariable path analysis. Ann Behav Med. 2017;51(2):321–326.
- Shi Z, Rundle A, Genkinger JM, et al. Distinct trajectories of moderate to vigorous physical activity and sedentary behavior following a breast cancer diagnosis: the pathways study. J Cancer Surviv. 2020;14(3):393–403. kesäkuuta 2020
- Steinhilper L, Geyer S, Sperlich S. Health behavior change among breast cancer patients. Int J Public Health. 2013;58(4):603–613.
- Brunet J, Taran S, Burke S, et al. A qualitative exploration of barriers and motivators to physical activity participation in women treated for breast cancer. Disabil Rehabil. 2013;35(24):2038–2045.
- Monteiro-Guerra F, Signorelli GR, Rivera-Romero O, et al. Breast cancer survivors’ perspectives on motivational and personalization strategies in mobile App-Based physical activity coaching interventions: Qualitative study. JMIR Mhealth Uhealth. 2020;8(9):e18867.
- Owusu C, Antognoli E, Nock N, et al. Perspective of older African-American and Non-Hispanic white breast cancer survivors from diverse socioeconomic backgrounds toward physical activity: a qualitative study. J Geriatr Oncol. 2018;9(3):235–242.
- Larsson IL, Jonsson C, Olsson AC, et al. Women’s experience of physical activity following breast cancer treatment. Scand J Caring Sci. 2008;22(3):422–429.
- Kampshoff CS, Jansen F, van Mechelen W, et al. Determinants of exercise adherence and maintenance among cancer survivors: a systematic review. Int.j.behav.nutr.phys.act. 2014;11(Journal Article):80.
- Tyrrell A, Keats M, Blanchard C. The physical activity preferences of gynecologic cancer survivors. Oncol Nurs Forum. 2014;41(5):461–469.
- Vehmanen L, Elomaa I, Blomqvist CP, et al. The effect of ovarian dysfunction on bone mineral density in breast cancer patients 10 years after adjuvant chemotherapy. Acta Oncol. 2014;53(1)(Journal Article):75–79.
- Vehmanen L, Sievanen H, Kellokumpu-Lehtinen P, et al. Five-year follow-up results of aerobic and impact training on bone mineral density in early breast cancer patients. Osteoporos Int. 2021;32(3):473–482.
- Penttinen H, Nikander R, Blomqvist C, et al. Recruitment of breast cancer survivors into a 12-month supervised exercise intervention is feasible. Contemp Clin Trials. 2009;30(5):457–463.
- Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381.
- Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 Compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43(8):1575–1581.
- Baranowski T, Smith M, Thompson WO, et al. Intraindividual variability and reliability in a 7-day exercise record. Medicine & Science in Sports & Exercise. 1999;31(11):1619–1622.
- Oja P, Laukkanen R, Pasanen M, et al. A 2-km walking test for assessing the cardiorespiratory fitness of healthy adults. Int J Sports Med. 1991;12(4):356–362.
- Aaronson NK, Ahmedzai S, Bergman B, et al. The european organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376.
- Sprangers MA, Groenvold M, Arraras JI, et al. The european organization for research and treatment of cancer breast cancer-specific quality-of-life questionnaire module: first results from a three-country field study. J Clin Oncol. 1996;14(10):2756–2768.
- Saarto T, Penttinen HM, Sievanen H, et al. Effectiveness of a 12-month exercise program on physical performance and quality of life of breast cancer survivors. Anticancer Res. 2012;32(9):3875–3884.
- van der Zee MD, van der Mee D, Bartels M, et al. JC. Tracking of voluntary exercise behaviour over the lifespan. Int J Behav Natr Phys Act. 2019;16(1):17.
- Ormel HL, van der Schoot GGF, Sluiter WJ, et al. Predictors of adherence to exercise interventions during and after cancer treatment: a systematic review. Psychooncology. 2018;27(3):713–724.
- Anderson AS, Caswell S, Wells M, et al. It makes you feel so full of life” LiveWell, a feasibility study of a personalised lifestyle programme for colorectal cancer survivors. Support Care Cancer. 2010;18(4):409–415.
- Courneya KS, Friedenreich CM. Physical activity and cancer control. Semin Oncol Nurs. 2007;23(4):242–252.
- Gjerset GM, Fossa SD, Courneya KS, et al. Interest and preferences for exercise counselling and programming among norwegian cancer survivors. Eur J Cancer Care (Engl). 2011;20(1):96–105.
- Pinto BM, Maruyama NC. Exercise in the rehabilitation of breast cancer survivors. Psychooncology. 1999;8(3):191–206.
- Pinto BM, Papandonatos GD, Goldstein MG. A randomized trial to promote physical activity among breast cancer patients. Health Psychol. 2013;32(6):616–626.
- Thorsen L, Courneya KS, Stevinson C, et al. A systematic review of physical activity in prostate cancer survivors: outcomes, prevalence, and determinants. Support Care Cancer. 2008;16(9):987–997.
- Cuevas BT, Hughes DC, Parma DL, et al. Motivation, exercise, and stress in breast cancer survivors. Support Care Cancer. 2014;22(4):911–917.
- Kampshoff CS, van Mechelen W, Schep G, et al. Participation in and adherence to physical exercise after completion of primary cancer treatment. Int J Behav Nutr Phys Act. 2016;13(1):100.
- Kalter J, Kampshoff CS, Chinapaw MJM, et al. Mediators of exercise effects on HRQoL in cancer survivors after chemotherapy. Med Sci Sports Exerc. 2016;48(10):1859–1865.
- Berger AM, Abernethy AP, Atkinson A, et al. NCCN clinical practice guidelines cancer-related fatigue. J Natl Compr Canc Netw. 2010;8(8):904–931.
- Poikonen-Saksela P, Kolokotroni E, Vehmanen L, et al. A graphical LASSO analysis of global quality of life, Sub scales of the EORTC QLQ-C30 instrument and depression in early breast cancer. Sci Rep. 2022;12(1):2112.
- Stricker CT, Drake D, Hoyer KA, et al. Evidence-based practice for fatigue management in adults with cancer: exercise as an intervention. Oncol Nurs Forum. 2004;31(5):963–976.
- Spence RR, Heesch KC, Brown WJ. Exercise and cancer rehabilitation: a systematic review. Cancer Treat Rev. 2010;36(2):185–194.