ABSTARCT
Background
There is an ongoing need to identify biomarkers for correct patient selection for immune-oncology treatments in metastatic renal cell carcinoma (mRCC). The aim of our study was to evaluate the prognostic role of elevated C-reactive protein (CRP) values and immune-related adverse events (irAEs) to indicate immune checkpoint inhibitors’ (ICIs) efficacy in nivolumab-treated mRCC patients.
Materials and methods
Data from 96 mRCC patients treated with nivolumab at Comprehensive Cancer Center, Helsinki University Hospital in a real-life setting were collected between 2006 and 2020 retrospectively. Patients’ baseline CRP, on-treatment (<12 weeks) CRP, and reported irAE association to median survival and outcome were analyzed using Kaplan–Meier and Cox regression.
Results
Patients with elevated baseline CRP were associated with worse overall survival (OS) and progression-free survival (PFS) when compared with normal baseline CRP. This significant correlation was also observed with patients with elevated on-treatment CRP. In multivariate survival analyses both elevated baseline and on-treatment CRP had shorter OS and PFS than patients with normal CRP: hazard ratio (HR) 2.84 (95% CI 1.48–5.42), HR 3.68 (95% CI 1.92–7.03) and PFS: HR 1.77 (95% CI 1.06–2.97), HR 2.88 (95% CI 1.75–4.73), respectively. A significant difference in OS was also seen between patients without irAE and with irAE during treatment. In multivariate survival analyses, patients without irAE had shorter OS HR 1.93 (95% CI 1.03–3.62) compared with patients with reported irAE.
Conclusions
Elevated baseline CRP, on-treatment CRP, and absence of irAE correlate with poor outcome in nivolumb-treated mRCC patients. These results suggest that monitoring CRP values as well as potential irAEs during treatment may be of use in clinical decision making.
Introduction
For the past few years, immune checkpoint inhibitors (ICIs) have made their way into routine clinical practice in treating metastatic renal cell carcinoma (mRCC) [Citation1]. ICIs nivolumab and pembrolizumab, in combination with tyrosine kinase inhibitors (TKIs) cabozantinib or axitinib, are recommended by European Society of Medical Oncology (ESMO) 2021 guidelines as first-line treatments for mRCC in all International Metastatic Database Consortium (IMDC) prognostic risk groups [Citation2]. Nivolumab in combination with anti-CTLA-4 inhibitor ipilimumab has shown an overall survival (OS) benefit compared with sunitinib and is approved as a first-line treatment among mRCC patients with intermediate or poor risk groups according to IMDC criteria [Citation2,Citation3]. Furthermore, single-agent nivolumab is recommended by ESMO 2021 guideline for mRCC patients previously treated with any front-line targeted therapy [Citation2].
Although ICIs have shown a significant improvement in OS and progression-free survival (PFS) over targeted therapies in mRCC, a large portion of patients do not respond to these treatments. In a registration study by Motzer et al., nivolumab showed an OS benefit of 25.0 months versus 19.6 months when compared with everolimus in 2–4th line mRCC patients. However, in this study, the nivolumab overall response rate (ORR) was only 25% [Citation4]. There is thus an interest in finding new, clinically applicable prognostic biomarkers to identify patients most likely to benefit from ICI treatment.
ICIs target the programmed cell death (PD-1) receptor and inhibit its interaction between PD-L1 and PD-L2 ligands. Normal interaction between PD-1 and PD-L1 or PD-L2 inhibits the cellular immune response [Citation5]. However, ICIs do not just target tumor-specific immune interactions but can overstimulate the immune response and induce a nonspecific autoimmunological activation. This nonspecific targeting may cause immune-related adverse events (irAEs) in ICI-treated patients [Citation6]. IrAEs can manifest in different organs or tissues in various severities. Most common irAEs reported are cutaneous, hepatic, gastrointestinal, and endocrinological adverse events [Citation7].
Although research has shown that ICIs have a lower incidence of adverse events compared with targeted therapies in mRCC, treatment-related adverse events of any grade still occurred in 79% of patients treated with nivolumab. Also grade 3 or 4 toxicities manifested in 19% of patients and irAEs led to treatment discontinuation in 9% of patients [Citation4]. Several studies have demonstrated that there is an association between outcome and irAEs among patients treated with ICIs [Citation8–10] and it has been postulated that irAEs may reflect treatment response in host’s immune system. [Citation11]
C-reactive protein (CRP) is an acute phase reactant, as it indicates systemic inflammation and activates innate immunity [Citation12]. Previous studies have shown the association between different systemic inflammatory factors (neutrophil-to-lymphocyte ratio, monocyte-to-lymphocyte ratio, CRP) and poor prognosis in several malignancies [Citation13–15], including mRCC [Citation16,Citation17]. CRP has been found to be a prognostic biomarker in ICI-treated melanoma [Citation18], non-small cell lung cancer patients [Citation19,Citation20], and lately also in mRCC [Citation21–24]. However, evidence regarding the role of CRP as a potential prognostic biomarker in mRCC remains limited and the potential association between baseline CRP level and outcome in ICI-treated mRCC patient needs further validation to be used in routine clinical practice.
Due to the pharmacological safety profile of ICI treatment and lack of any current prognostic biomarkers for ICI treatment efficacy, there is an ongoing and high clinical interest to improve patient selection. The aim of our study was to find clinically useful, inflammatory or immunological factors related to outcome of ICI treatment in mRCC patients and to evaluate the prognostic role of elevated CRP values and irAEs to indicate ICIs’ efficacy in mRCC patients.
Material and methods
All clinicopathological features, adverse events (AE), treatment history, and laboratory values were collected from electrical patient medical records.
Patients and treatment
A total of 480 patients were treated and diagnosed with mRCC at Comprehensive Cancer Center, Helsinki University Hospital between 18 October 2006 and 31 December 2020. Of these patients, a total of 96 patients were treated with nivolumab as second or later line therapy and all patients with required baseline and on-treatment data were included in our study. An investigational agent was given to seven (6.3%) patients and ipilimumab to six (6.3%) patients in combination with nivolumab. Prior to treatment initiation, nivolumab treatment was evaluated and recommended by a multidisciplinary team meeting. Patients with regular corticosteroid medication or history of autoimmune diseases were not eligible to be treated with nivolumab. All patients received nivolumab 480 mg intravenously every four weeks or 3 mg/kg every two weeks. Nivolumab was given until progressive disease (PD), death of any cause or development of AEs that required treatment discontinuation according to the treating physician.
Patients were characterized according to the IMDC prognostic risk group (favorable, intermediate or poor) based on time from diagnosis to treatment less than a year, Karnofsky performance status, baseline hemoglobin, neutrophils, thrombocytes, and albumin-corrected calcium levels.
Assessment of tumor response
All patients’ follow-up was carried out according to standard mRCC treatment guidelines. Response to treatment was assessed by computed tomography (CT) scan at 8–12-week intervals depending on patients’ condition. Treatment efficacy was evaluated by experienced cancer radiologists and defined by Response Evaluation Criteria in Solid Tumors (RECIST) v. 1.1.
Assessment of adverse event
All adverse event data (grade, beginning and resolving dates and treatments used) were collected from hospital case records. On-treatment-related AEs were evaluated by the treating physician. AEs were defined by Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Immune-related adverse events included in this study were cutaneous (rash, eczema, pruritus), respiratory (pneumonitis), gastrointestinal (colitis/diarrhea), hepatic (increased ALT or AST), endocrinopathies (type I diabetes, hypothyreosis, thyroiditis, hypophysitis), pancreatitis, arthritis, cardiac toxicities (pericarditis, total AV block), neurological toxicities, stomatitis, and gastritis. The collected irAEs were selected according to ESMO clinical practice guideline. All irAEs are summarized in .
Table 1. Frequency of immune related adverse events.
Assessment of C-reactive protein
Baseline blood values, including CRP, were collected 115 days before first nivolumab infusion. On-treatment CRP value was determined as the lowest CRP value within 12 weeks of treatment initiation. A CRP level of >10 mg/l was considered elevated according to standard hospital cutoff level.
Statistics
The study population consisted of 96 consecutive patients. Patient characteristics were described overall and by baseline CRP status. Median follow-up time for patients alive was assessed using Schemper’s method. The association of CRP values, irAEs and other clinical variables was assessed using the Mann–Whitney U test for continuous data and the Chi-squared test for categorical data. The primary endpoint in the study was (i) overall survival defined as the time from treatment initiation to death, whatever the cause. Secondary endpoints were (ii) progression-free survival defined as the time from treatment initiation to the first event (tumor progression or death from any cause) and (iii) clinical benefit rate defined as complete response (CR), partial response (PR) or stable disease (SD) as overall best response according to RECIST 1.1. The Kaplan–Meier method was used to estimate the median survival times with 95% confidence intervals (CI) for both OS and PFS, censoring the patients who were alive or had no disease progression at the last follow-up visit. Log-rank test was used to demonstrate p-values.
Univariate Cox proportional hazard model was used to assess the association between clinical variables and OS and PFS. Multivariate analyses for OS and PFS were performed using Cox proportional hazards models adjusted for age, IMDC risk classification, irAEs and baseline, and on-treatment CRP values. Due to a strong correlation of irAE, baseline, and on-treatment CRP values, multivariate analyses were performed separately. The proportional hazards assumption was assessed graphically obtaining plots of (log(-logS(t)) versus time and Schoenfeld residuals versus time.
A multivariate logistic regression model was used to investigate the effects of age, IMDC risk classification, and on-treatment-CRP and irAEs on the CBR. The results are expressed as ORs with 95% confidence interval.
A landmark survival analysis was made to avoid bias from longer treatment. Landmark was set at three months (n = 84 patients at 3 months) after the first initiation. Patients were excluded (n = 12) if the treatment time was shorter than three months.
Concordance index (c-index) was computed to demonstrate the discrimination power of baseline CRP and on-treatment CRP for both OS and PFS. Also, to show the improvement of accuracy when baseline CRP or on-treatment CRP was added in IMDC risk classification. C-index was expressed with standard error (SE).
All statistical tests were two-sided and p-values < .05 were considered as statistically significant. Analysis was performed using IBM SPSS Statistics for Windows (version 25.0, Armonk, NY, USA, IBM Corp.), excluding c-index, which was calculated using Statistical Analysis Software SAS/STAT.
Results
Patient characteristics
A total of 51 patients (53.1%) received nivolumab as second, 27 patients (28.1%) as third and 18 patients (18.7%) as fourth or later treatment line. Patients received nivolumab therapy after one to eight lines of previous TKI therapy. Mammalian target of rapamycin inhibitor everolimus was given to 15.6% of patients before nivolumab. After nivolumab treatment, 56.3% received another systemic anti-cancer therapy. Median follow-up time of patients alive was 35.2 months and the median duration on nivolumab treatment was 12 weeks (range 1–212 weeks). At the time of data cutoff, nine (9.4%) patients continued nivolumab treatment. A total of 61 (63.5%) patients stopped treatment due to progression and 26 (27.1%) patients without progression (18 due to irAEs, eight for other reasons). Patient characteristics are depicted in .
Table 2. Patients’ characteristics overall and by baseline CRP status.
The median OS and PFS for the whole patient cohort were 21.5 months (95% CI 12.8–30.2) and 3.3 months (95% CI 1.6–5.0), respectively. According to IMDC risk groups, median OS was 21.6 months (95% CI 11.3–31.9) and 11.9 months (95% CI 7.3–16.5) for intermediate and poor risk groups, respectively. For patients in the favorable risk group, OS was not reached (NR). The median PFS was 4.6 months (95% CI 1.3–7.9), 1.5 months (95% CI 0.6–6.7), and 0.3 months (95% CI 1.5–2.6) for favorable, intermediate, and poor IMDC risk groups, respectively.
The overall response rate per RECIST criteria was 22.9% (n = 22). A total of 46 (47.9%) patients had PD as their best overall response. The clinical benefit rate (CBR) was 52.1%, consisting of 28 stable disease (SD) and 20 partial response (PR) and two complete response (CR) who remained in remission at the time of data cutoff.
CRP and outcome
In our study, 42 patients had elevated baseline CRP. Of these patients, 59.3% received nivolumab as second, 25.9% as third, and 14.9% as fourth or later line treatment. Significantly more males had elevated baseline CRP as compared with females () and baseline CRP was statistically significantly associated with IMDC risk groups. There were no statistically significant associations with other patient characteristics.
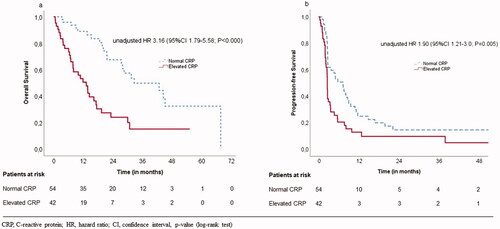
In univariate analysis (), patients with elevated baseline CRP had worse OS when compared with normal baseline CRP 13.2 months (95% CI 7.8–18.7) versus 32.7 months (95% CI 13.6–51.8), p < .001 as well as PFS: 2.5 months (95% CI 2.4–2.6) versus 7.2 months (95% CI 4.1–10.3), p = .005, respectively; ).
Patients with elevated on-treatment CRP also had both worse OS and PFS than patients with normal on-treatment CRP: OS 13.2 months (95% CI 8.2–18.3) versus 32.7 months (95% CI 16.7–48.6), p < .001 and PFS 2.4 months (95% CI 1.9–2.8) versus 7.2 months (95% CI 4.5–10.0), p = .005.
A landmark analysis was utilized, and landmark was set at three months after the first initiation. Patients with normal on-treatment CRP values had improved OS than patients with elevated on-treatment CRP values: median of 29.7 months (95% CI 13.0–46.4) versus 10 months (95% CI 8.5–13.2), p = .001. For PFS the landmark analysis results did not remain significant.
irAE and outcome
Altogether, 34 (35.4%) patients reported any (grade 1–4) irAEs and 18 (18.8%) patients had to discontinue the treatment due to irAEs. Altogether, nine (9.4%) patients reported grade ≥3 irAEs. All of these patients were treated with either oral or intravenous corticosteroid to resolve the irAEs. The most frequent irAE was cutaneous toxicity (14.6%; ).
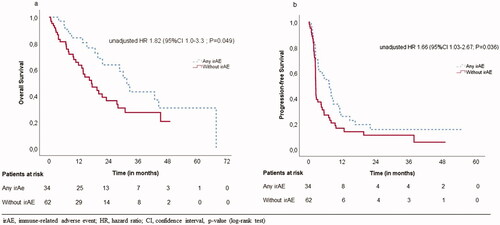
The CBR among patients with any irAEs was 70.6% versus 41.9% among patients without irAEs (p = .010).
In univariate survival analysis, we found that patients without irAEs during treatment had significantly worse OS and PFS as compared with patients with irAEs: OS 17.2 months (95% CI 10.9–23.5) versus 31.5 months (95% CI 26.0–36.9), p = .046 and PFS 2.6 months (95% CI 2.4–2.7) versus 7.5 months (95% CI 4.1–10.8) p = .033, respectively; ).
Since irAE is a time-varying covariate, landmark analysis was made to avoid immortal time bias. However, these results did not remain statistically significant. To demonstrate patients’ risk of dying and association with experienced irAE without time dependency, we used Fisher’s exact test at three months and six months. It demonstrated that all patients who had irAE were alive, but 87.9% of patients who did not have any irAEs were alive after three months of initiation (p = .046). Also, 96.9% of patient, who experienced irAE were alive, but 82.7% of patients who did not have any irAE were alive after six months of first initiation (p = .081).
Multivariate analyses
In a multivariate model adjusted for IMDC classification and age >65 years, elevated baseline CRP was associated with shorter OS (HR 2.84 95% CI 1.48–5.42) and PFS (HR 1.77 95% CI 1.06–2.97). Elevated on-treatment CRP was associated with shorter OS (HR 3.68 95% CI 1.92–7.03) and PFS (HR 2.88 95% CI 1.75–4.73). Patients without irAE during treatment were associated with shorter OS (HR 1.93 95% CI 1.03–3.62), but there was no significant association with PFS (p = .082). Baseline CRP and on-treatment CRP value had a strong correlation (Cramer's V = 0.64). Baseline and on-treatment CRP had a weaker correlation with irAE (Cramer’s V = 0.30, 0.23), but this was statistically significant (p = .005, 0.030), therefore Cox regression multivariate analyses were made separately. The results of these multivariate PFS and OS survival analyses are shown in .
Table 3. Multivariate survival analysis.
A multivariate logistic regression analysis was performed to determine the adjusted effects of irAEs and on-treatment CRP on CBR of univariate analyses. Logistic regression was not determined for baseline CRP, since there was no significant correlation between baseline CRP and CBR (p = .150). In a model adjusted for age, gender, BMI >25 kg/m2, and IMDC prognostic group, patients without irAEs were three times more likely to have PD as their best response when compared with patients with any irAEs during treatment (OR 3.47 95% CI 1.25–9.66). Patients with elevated on-treatment CRP were three times more likely to have PD as their best response when compared with patients with normal on-treatment CRP (OR 3.62 95% CI 1.34–9.80).
Concordance index
To ascertain the accuracy in discriminating patient risk, the concordance index (c-index) was computed for baseline CRP and on-treatment CRP for OS: 0.65 (SE 0.059) and 0.65 (SE 0.027) and for PFS: 0.60 (SE 0.032) and 0.64 (SE 0.027), respectively. The c-index improved when on-treatment CRP or baseline CRP were added to IMDC risk classification compared with IMDC risk classification alone for OS: 0.71 (SE 0.051), 0.70 (SE 0.05) versus 0.63 (SE 0.04), respectively, and for PFS on-treatment CRP 0.66 (SE 0.03), baseline CRP 0.62 (SE 0.05) versus 0.57 (SE 0.03), respectively showing increased prognostic accuracy.
Subgroup analyses
To evaluate the relationship of baseline and on-treatment CRP values in further detail, we performed a subgroup analysis among patients with normal and elevated CRP values at baseline and during treatment. Patients with normal CRP values throughout treatment had significantly longer OS and PFS as compared with persistently elevated CRP values; OS 42.7 months (95% CI 26.8–58.7) versus 10.0 months (95% CI 4.8–15.2) p < .001) and PFS 7.6 months, (95% CI 3.8–11.4) versus 2.4 months (95% CI 1.9–2.8), p < .001.
In addition, a shift from elevated baseline CRP values to normal on-treatment values was associated with better OS as compared to patients with persistently elevated CRP values: OS 19.4 months (95% CI 2.5–36.3) versus 10.0 months (95% CI 4.8–15.2), p < .001.
Discussion
ICI treatments have an established role in standard treatment of mRCC both in first and second line. Despite profound efforts to find biomarkers for treatment selection, good ones still have not been identified, even though data from prospective settings exist. As an example, Motzer et al. sought to distinguish patients benefitting from ICI-TKI combination as first-line therapy by analyzing immune cell distributions and genetic alterations. Although they could not identify a significant interaction with PFS and CD8+ T cells or PD-L1 expression in ICI-TKI-treated patients, this does not necessarily mean that they are irrelevant but instead that other contributing factors exist [Citation25].
Furthermore, in a prospective follow-up study of nivolumab plus ipilimumab versus sunitinib, a positive trend was seen between irAEs and OS in nivolumab plus ipilimumab-treated patients [Citation26]. The potential mechanisms and association between irAEs and anti-tumor reaction have been studied previously in different malignancies treated with ICIs [Citation11]. However, it is still unclear whether it is the same immune reaction causing irAEs that also targets the tumor. In a case report where a severe fatal myocarditis developed after nivolumb plus ipilimumb treatment, a robust accumulation of T-cells and macrophages were found in the myocardium, identical to those in the patient’s tumor. Theories suggest that there is a low selectivity among tumor-reactive T-cells resulting in targeting normal tissue as well. This in turn leads to development of both irAEs and anti-tumor reactions [Citation27]. Another pathway suggested is that preexisting tolerated self-reactive T-cells are deregulated in the periphery, leading to activation and upregulation of both the anti-tumor and an autoimmune reaction [Citation28].
Our study is in line with previous studies with ICI-treated melanoma [Citation29] and small cell lung cancer [Citation30], where immune reaction and irAEs are seen to correlate with better outcome. Interestingly, different malignancies have reported to have different irAE profiles even when treated with the same ICI. For example, melanoma patients treated with nivolumab developing a cutaneous irAE, vitiligo, were associated with better OS [Citation31], but this same correlation or occurrence has not been reported in nivolumab-treated mRCC or small cell lung cancer patients. This histology-specific reaction of ICIs may be explained by different tumor microenvironments and cell activities enhanced by nivolumab. As Choueiri et al. showed in prospective setting that nivolumab has immunomodulatory effects in tumor microenvironment, as tumor-associated lymphocytes, CD3 and CD8 levels increased significantly during treatment. This suggests that nivolumab reverses T-cell exhaustion and boosts T-cell-mediated antitumor immune activity in tumor microenvironment, which might be causing different adverse events [Citation32]. Higher understanding regarding ICIs’ immune activation in tumor microenvironment and in circulation is needed to find out the relations between different irAE pathways and biological response.
Our results demonstrate that both baseline and on-treatment CRP values are independently associated with outcome. CRP also acts as a dynamic biomarker; normalization of CRP values during treatment indicated prolonged OS as compared with patients with persistently elevated CRP values. Although the association between systemic inflammation, innate immunity, and cancer is well established, the intricate mechanisms behind these connections remain unclear [Citation33]. Circulating blood cells like neutrophils, platelets, and hemoglobin as well as CRP are associated with systemic inflammation. Neutrophils, platelets, and hemoglobin are included in the widely used prognostic IMDC (Heng) risk classification for mRCC [Citation34]. Our findings demonstrate that in addition to IMDC risk classification, baseline, and on-treatment CRP, a readily available, inexpensive, and reproducible laboratory parameter could be utilized to improve prognostication among mRCC patients. This study also adds to the growing evidence of irAEs as biomarkers for immune activity and treatment response and in ICI-treated patients.
In conclusion, our real-life retrospective data from a large Nordic comprehensive cancer center show that baseline and on-treatment CRP values are independent prognostic biomarkers for overall survival as well as for progression-free survival and that irAEs were associated with response to treatment among mRCC patients treated with nivolumab. In addition to the potential application of baseline CRP as a prognostic biomarker, these results suggest that monitoring CRP values as well as potential irAEs during treatment may be of use in clinical decision making.
Limitations
Our study was a single center design with relatively small number of patients treated with ≥2nd line nivolumab. Potential limitations were also its exploratory and retrospective nature. Our cohort represents a heterogeneous group of mRCC patients including different histology and several previous treatment lines. IrAEs were collected from patient history retrospectively so mild or transient irAEs might not been reported. Events were individually physician – determined and reporting can vary in between patients and physicians. Multiple adverse events were reported in a single patient which makes interpreting the data more complex. The timing onset of irAEs varies widely and it may be difficult to distinguish irAEs from other symptoms caused by cancer itself. As irAE and on-treatment CRP are time-varying covariates and are affected by immortal bias, three-month landmark analyses were made. Results for irAE and on-treatment PFS did not remain statistically significant which is probably due to the small size of our cohort.
Disclosure statement
K. Peltola has a consultant or advisory role for MSD Oncology, Lilly, Pfizer, Bristol-Myers Squibb, Novartis, Ipsen, Roche, stock ownership of Faron Pharmaceuticals and has received travel support from Bristol-Myers Squibb and Roche. P. Bono has received honoraria from MSD, Pfizer, Bristol-Myers Squibb, Novartis, Ipsen, Oncorena, TILT Biotherapeutics, Faron Pharmaceuticals, Eisai and Herantis Pharma, and stock ownership of TILT Biotherapeutics and Terveystalo. The other authors report no conflict of interest to declare.
Data availability statement
The data that support the findings of this study are available from the corresponding author, E. Kankkunen, upon reasonable request.
References
- Mo D-C, Huang J-F, Luo P-H, et al. Combination therapy with immune checkpoint inhibitors in advanced renal cell carcinoma: a meta-analysis of randomized controlled trials. Clin Immunol. 2021;232:108876.
- Powles T, ESMO Guidelines Committee. Electronic address: [email protected] Recent eUpdate to the ESMO clinical practice guidelines on renal cell carcinoma on cabozantinib and nivolumab for first-line clear cell renal cancer. Ann Oncol. 2021;32(3):422–423.
- Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–1290.
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–1813.
- Harshman LC, Drake CG, Choueiri TK. PD-1 blockade in renal cell carcinoma: to equilibrium and beyond. Cancer Immunol Res. 2014;2(12):1132–1141.
- Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33(17):1974–1982.
- Ornstein MC, Garcia JA. Toxicity of checkpoint inhibition in advanced RCC: a systematic review. Kidney Cancer. 2017;1(2):133–141.
- Maher VE, Fernandes LL, Weinstock C, et al. Analysis of the association between adverse events and outcome in patients receiving a programmed death protein 1 or programmed death ligand 1 antibody. J Clin Oncol. 2019;37(30):2730–2737.
- Martini DJ, Goyal S, Liu Y, et al. Immune‐related adverse events as clinical biomarkers in patients with metastatic renal cell carcinoma treated with immune checkpoint inhibitors. The Oncologist. 2021;26(12):1017–1025.
- Paderi A, Giorgione R, Giommoni E, et al. Association between immune related adverse events and outcome in patients with metastatic renal cell carcinoma treated with immune checkpoint inhibitors. Cancers. 2021;13(4):860.
- Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunotherapy Cancer. 2019;7(1):306.
- Du Clos TW. Function of C-reactive protein. Ann Med. 2000;32(4):274–278.
- Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-Lymphocyte ratio in solid tumors: a systematic review and meta-Analysis. JNCI. 2014;106(6):dju124-dju.
- Bowen RC, Little NAB, Harmer JR, et al. Neutrophil-to-lymphocyte ratio as prognostic indicator in gastrointestinal cancers: a systematic review and meta-analysis. Oncotarget. 2017;8(19):32171–32189.
- Ethier J-L, Desautels D, Templeton A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res. 2017;19(1):2.
- Pilskog M, Beisland C, Akslen LA, et al. Predictive value of C-reactive protein in patients treated with sunitinib for metastatic clear cell renal cell carcinoma. BMC Urol. 2017;17(1):74.
- Takamatsu K, Mizuno R, Omura M, et al. Prognostic value of baseline serum C-reactive protein level in intermediate-risk group patients with metastatic renal-cell carcinoma treated by first-line vascular endothelial growth factor–targeted therapy. Clin Genitourin Cancer. 2018;16(4):e927–e33.
- Laino AS, Woods D, Vassallo M, et al. Serum interleukin-6 and C-reactive protein are associated with survival in melanoma patients receiving immune checkpoint inhibition. J Immunother Cancer. 2020;8(1):e000842.
- Adachi Y, Tamiya A, Taniguchi Y, et al. Predictive factors for progression‐free survival in non‐small cell lung cancer patients receiving nivolumab based on performance status. Cancer Med. 2020;9(4):1383–1391.
- Riedl JM, Barth DA, Brueckl WM, et al. C-Reactive protein (CRP) levels in immune checkpoint inhibitor response and progression in advanced non-small cell lung cancer: a bi-center study. Cancers. 2020;12(8):2319.
- Bilen MA, Dutcher GMA, Liu Y, et al. Association Between pretreatment neutrophil-to-lymphocyte ratio and outcome of patients with metastatic renal-cell carcinoma treated with nivolumab. Clinical Genitourinary Cancer. 2018;16(3):e563–e75.
- Ishihara H, Tachibana H, Takagi T, et al. Predictive impact of peripheral blood markers and C-Reactive protein in nivolumab therapy for metastatic renal cell carcinoma. Target Oncol. 2019;14(4):453–463.
- Roussel E, Kinget L, Verbiest A, et al. C-reactive protein and neutrophil-lymphocyte ratio are prognostic in metastatic clear-cell renal cell carcinoma patients treated with nivolumab. Urol Oncol. 2021;39(4):239.e17–e25.
- Suzuki K, Terakawa T, Furukawa J, et al. C-reactive protein and the neutrophil-to-lymphocyte ratio are prognostic biomarkers in metastatic renal cell carcinoma patients treated with nivolumab. Int J Clin Oncol. 2020;25(1):135–144.
- Motzer RJ, Robbins PB, Powles T, et al. Avelumab plus axitinib versus sunitinib in advanced renal cell carcinoma: biomarker analysis of the phase 3 JAVELIN renal 101 trial. Nat Med. 2020;26(11):1733–1741.
- Motzer RJ, Escudier B, McDermott DF, et al. Survival outcomes and independent response assessment with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma: 42-month follow-up of a randomized phase 3 clinical trial. J Immunother Cancer. 2020;8(2):e000891.
- Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375(18):1749–1755.
- Yoest J. Clinical features, predictive correlates, and pathophysiology of immune-related adverse events in immune checkpoint inhibitor treatments in cancer: a short review. Immunotargets Ther. 2017;6:73–82.
- Indini A, Di Guardo L, Cimminiello C, et al. Immune-related adverse events correlate with improved survival in patients undergoing anti-PD1 immunotherapy for metastatic melanoma. J Cancer Res Clin Oncol. 2019;145(2):511–521.
- Ricciuti B, Genova C, De Giglio A, et al. Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: long-term outcomes from a multi-institutional analysis. J Cancer Res Clin Oncol. 2019;145(2):479–485.
- Teulings H-E, Limpens J, Jansen SN, et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33(7):773–781.
- Choueiri TK, Fishman MN, Escudier B, et al. Immunomodulatory activity of nivolumab in metastatic renal cell carcinoma. Clinical Cancer Research. 2016;22(22):5461–5471.
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867.
- Heng DYC, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated With vascular endothelial growth factor–targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27(34):5794–5799.