Introduction
The journey of a thousand miles in lung cancer therapy began with the discovery of actionable genomic alterations. The two most well described molecular alterations are the epidermal growth factor receptor (EGFR) mutation and ALK fusion [Citation1,Citation2]. Initially, we thought that these oncogenic drivers are mutually exclusive, i.e. only a single driver per tumor. However, we now know that tumors may harbor more than one molecular driver, including concurrent EGFR and ALK alterations [Citation3–9]. We present a case series of patients with NSCLC with concurrent EGFR and ALK alterations.
Materials and methods
General study details
This was a retrospective analysis of patients enrolled in the Institutional Ethics Committee-approved Lung Cancer Audit study conducted in the Department of Medical Oncology, at the Tata Memorial Hospital, in Mumbai, India. Written informed consent was obtained. The study was registered with the Clinical Trials Registry-India (CTRI/2013/01/003335). The study was conducted according to the ethical guidelines established by the Declaration of Helsinki. No funding was utilized for this study.
Participants
Patients with advanced NSCLC, whose tumors were noted to have a mutation in EGFR and a concurrent ALK fusion at baseline were included. There were no exclusion criteria.
Variables
Our primary objective was to evaluate the overall survival (OS) of patients with NSCLC and concurrent EGFR and ALK alterations. Secondary objectives were describing the clinicopathologic features, pattern of therapy, toxicities, progression-free survival (PFS) and resistance mechanisms.
Methodology
We identified the patients from the lung cancer audit, molecular laboratory records and the prospectively maintained database of the weekly institutional Molecular Tumor Board meetings. Details were supplemented from the electronic medical records. Patients who had not visited the hospital in the past three months for follow-up were telephonically contacted.
Statistics
There was no sample size calculation for this study. We included all eligible patients. The data were analyzed in the Statistical Program for the Social Sciences, Version 23.0. PFS was defined from the date of start of oral tyrosine kinase inhibitors (TKI) (or the start of chemotherapy in the patient who received only chemotherapy), to the date of progression on that TKI, either objective or subjective, or death without disease progression. OS was calculated from the date of diagnosis in patients with upfront metastatic disease, or progression for patients who received initial curative intent therapy, to the date of death from any cause. For patients who were lost to follow-up, we added three months to the last date of follow-up and considered that the events of progression/death occurred on that date (worst case scenario) [Citation10]. Survival was calculated using Kaplan-Meier method [Citation11]; comparison of survival was done using log-rank test. The impact of various factors on survival, including the performance status (ECOG PS 0–2, vs 3–4), type of EGFR mutation (sensitizing vs resistance), receipt of EGFR-directed, and of ALK-directed therapy was evaluated using Cox proportional hazard model [Citation12,Citation13]. Tumor response was characterized using Response Evaluation Criteria in Solid Tumors, version 1.1; toxicities were graded according to Common Terminology Criteria for Adverse Events (CTCAE), version 5.0.
Results
Baseline clinicodemographic features
Between 2013 and 2022, we identified 28 patients with concurrent EGFR and ALK alterations. We excluded four patients, two of whom had ALK positivity at baseline and were noted at progression to have concurrent ALK and EGFR alterations; one patient had weak ALK positivity on IHC but repeat ALK IHC was negative, and one patient had a sensitizing EGFR mutation at baseline and concurrent EGFR mutation and ALK fusion at progression. Thus, there were 24 patients in our case series. The clinicodemographic and disease-related details are provided in . All patients had Stage IV disease; one patient initially presented with locally advanced Stage III disease and received radical concurrent chemoradiotherapy; this patient progressed a year later with bilateral lung nodules.
Table 1. Demographic and clinicopathologic features of patients with dual EGFR and ALK positivity.
Metastases
The commonest extrathoracic region of metastasis was the abdomen; retroperitoneal/intraabdominal lymph nodes in eight patients (33%), liver in three (13%), adrenal in four (17%), omentum in two (8%), and ascites in two (8%). Brain metastases were detected at baseline and at progression in three patients (13%) each.
Molecular details
Testing was performed at baseline for 22 patients; two remaining patients were diagnosed and received first-line palliative chemotherapy elsewhere. They presented to us at progression; baseline tissue cell blocks were not available, and baseline molecular testing had not been performed. Therefore, the molecular testing at first progression was considered as the baseline. Testing for ALK was by IHC in all patients; 100% tested positive on IHC. ALK positivity was confirmed by FISH in one patient. Testing for EGFR was by RT-PCR in all patients. Molecular testing details are provided in Supplementary Table 1.
Therapy
Details of individual patients’ disease course and therapy are provided in Supplementary Table 2. First-line therapy consisted of chemotherapy (platinum-based combination) in five patients (21%), oral EGFR TKI in seven (29%), oral ALK-directed TKI in five (21%), and concurrent EGFR and ALK inhibitor in eight (33%). Considering the therapy provided during the disease course, 14 patients (58.3%) received both EGFR- and ALK-directed therapy, and 10 (41.7%) received therapy directed at either EGFR (n = 6, 25%), ALK (n = 3, 13%) or neither (n = 1 [4%] received only chemotherapy). Of the six patients who received sequential EGFR- and ALK-directed therapies, five received EFGR-directed therapy first followed by an ALK inhibitor at progression; in one patient, the sequence was an ALK inhibitor first followed by EGFR-directed therapy at progression.
Outcomes
Median follow-up in surviving patients was 32 months (95% CI, 27.97–36.03). Of the 21 patients who had an event for PFS, six (29%) did not receive second-line therapy because of poor PS (two patients, 10%), subjective progression at home and inability to return to hospital (2, 10%), defaulted/lost to follow-up (2, 10%). Summary of the disease outcomes is provided in Supplementary Table 3.
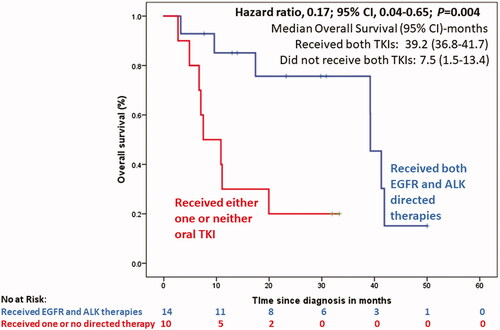
At a median follow-up of 32 months (95% CI, 27.8–36.2), the median OS in the 14 patients who received both EGFR- and ALK-directed therapies (sequentially or concurrently) was 39.2 months (95% CI, 36.75–41.7), compared to 7.5 months (95% CI, 1.59–13.4) in the 10 patients who received only one or neither of the targeted therapies against EGFR and ALK; HR, 0.17; 95% CI, 0.044–0.649; p = 0.004 (, Supplementary Figure 1).
Figure 1. Overall survival of patients with advanced NSCLC with dual EGFR and ALK drivers, based on whether the patients received both EGFR- and ALK-directed therapies (sequentially or concurrently) versus whether the patients received only one oral TKI or no TKI at all.
In a multivariate Cox regression analysis, the only factor significantly associated with prolongation of OS was the receipt of ALK-directed therapy; HR, 0.045; 95% CI, 0.008–0.264; p = 0.001 (Supplementary Table 4).
Toxicity
Grade ≥3 toxicities were similar in the concurrent EGFR- and ALK-inhibitor cohort (3/8, 38%), the EGFR-inhibitor cohort (3/6, 50%; p = 1.0) and the ALK inhibitor cohort (3/7, 43%; p = 0.49). Dose holds and dose reductions were required in three (43%) and two (29%) patients on ALK-inhibitors (n = 7); one (17%) and no patient on EGFR-inhibitors (n = 6); and three (38%; for skin rash in one, diarrhea in two) and one (13%) patient on concurrent EGFR- and ALK- inhibitors. Detailed toxicities are provided in Supplementary Table 5.
Resistance
Repeat histopathologic evaluation was performed in nine patients at progression. Molecular evaluation consisted of ALK testing by IHC in three samples (all were positive), confirmed by FISH in one; EGFR RT-PCR in three patients (all were wild type), and next generation sequencing (NGS) in three (two patients: ALK fusion detected, EGFR wild type; one patient: ALK fusion detected along with mutations in TP53 and SMAD4).
Discussion
In our case series of 24 patients with advanced NSCLC and concurrent EGFR and ALK alterations at baseline, patients were young (median age, 46 years), and over half (56%) were never-smokers. Adenocarcinoma was the commonest histology (96%); the disease had a predilection for intraabdominal metastases in 33%. Administering both EGFR- and ALK-directed therapies, either sequentially or concurrently, was paramount to optimizing outcomes; the median OS in patients who received only one or neither targeted therapy was 7.5 months (95% CI, 1.59–13.4) compared to 39.2 months (95% CI, 36.75–41.7) in those who received both targeted agents; HR, 0.17; 95% CI, 0.044–0.649; p = 0.004. This underlines the importance, both of testing for biomarkers in advanced NSCLC, and delivering appropriate targeted therapies directed against the identified molecular targets.
It was a long-held viewpoint that driver mutations are mutually exclusive [Citation14]. However, with the use of increasingly complex and widespread molecular testing for lung cancer, we now recognize that is not always true. Zhao and colleagues reported that approximately 1.5% of patients with NSCLC harbored co-occurring dual driver mutations that were potentially targetable. The patients with co-occurring driver mutations were more likely to be women (70%), nonsmokers (76%), and have tumors with adenocarcinoma histology (89%), similar to what we noted in our cohort. EGFR was the commonest gene to be associated with co-occurring alterations, which included MET, ERBB2, KRAS, ALK and other rarer mutations. The PFS of the patients with mutant EGFR and a co-occurring driver mutation was significantly shorter than that of patients with an exclusive EGFR mutation; median PFS, 5.4 vs 10.5 months; HR, 1.94, 95% CI, 1.16–3.25, p = 0.0042).[Citation15] Liu et al. also reported that patients with co-occurring EGFR and ALK alterations treated with EGFR-directed oral TKIs had a significantly shorter PFS (6 months) compared to patients with non-EGFR/ALK co-alterations (15 months), p = 0.046 [Citation8]. We also noted poor PFS (median PFS 6.1 months; 95% CI, 2.56–9.73) in our patients with dual EGFR and ALK alterations; the median PFS for EGFR-directed oral TKI therapy (in patients who received sequential EGFR- and ALK-directed therapy) was dismal at 3.3 months (95% CI, 0.64–5.86) which is lower than that reported in our earlier studies [Citation16,Citation17]. Thus, patients with EGFR and ALK dual molecular drivers appear to have a significantly poorer PFS than patients with exclusive targetable driver alterations.
Some authors have suggested that initial therapy with an EGFR oral TKI may be a good choice in patients with lung cancer and dual actionable mutations [Citation5,Citation6], while others have reported that upfront ALK inhibitors lead to the best efficacy.[Citation7] We initially targeted the co-occurring mutations sequentially, with the choice of the initial oral TKI left to the discretion of the treating physician. However, as our understanding of precision oncology evolved, we adopted the approach of upfront dual EGFR- and ALK- targeted therapies. Concurrent therapy was not excessively toxic, and although there was no clear evidence of enhanced efficacy over sequential EGFR- and ALK-directed therapies, the advantage was that patients could get exposed to both targeted agents. When using sequential targeted therapies, we found that approximately 30% of the patients did not receive second line therapy, due to various reasons. The mechanisms of resistance have been well described, both in EGFR-mutant as well as in ALK-rearranged lung cancers [Citation18,Citation19]. Some possible mechanisms of resistance in our cohort of patients included the clearance of EGFR mutation, histologic change to adenosquamous carcinoma, and mutations in TP53 and SMAD4.
Our study was limited by the small sample size, and the heterogenous treatment given to the patients. The targeted therapies consisted predominantly of first-generation oral TKIs; because many of the patients were started on therapy prior to the availability of data for the frontline use of third generation oral TKIs, and financial constraints. Imaging of the central nervous system was not performed in asymptomatic patients; thus, we were unable to determine the true incidence of brain metastases. We did not perform NGS at baseline and were unable to perform repeat biopsies in all patients at progression. Testing for ALK was by IHC [Citation20,Citation21]. Patient follow-up was performed at 3- to 6-month intervals, as per the institutional protocol, which may have impacted the PFS data. As our study was conducted in the midst of the global COVID-19 pandemic, several patients defaulted, due to inability to reach our hospital [Citation22–24], and 17% patients were lost-to-follow-up [Citation25]. In order to minimize the risk of bias and over-estimation of outcomes, we used the worst-case scenario to analyze survival data.
In conclusion, the dominant driver in NSCLC with dual EGFR/ALK alterations is ALK; targeting both molecular drivers concurrently is an attractive therapeutic option.
Supplemental Material
Download MS Word (44.1 KB)Supplemental Material
Download MS Word (164.9 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, [KP], upon reasonable request.
References
- Rajendra A, Noronha V, Joshi A, et al. Epidermal growth factor receptor-mutated non-small-cell lung cancer: a primer on contemporary management. Cancer Res Stat Treat. 2019;2(1):36–53.
- Prabhash K, Vora A, Limaye S, et al. Treatment of advanced non-small-cell lung cancer: First line, maintenance, and second line– Indian consensus statement update (under the aegis of Lung Cancer Consortium Asia, Indian Cooperative Oncology Network, Indian Society of Medical and Pediatric Oncology, Molecular Oncology Society, and Association of Physicians of India). Cancer Res Stat Treat. 2021;4(2):279–314.
- Talreja VT, Noronha V, Joshi A, et al. Coexistence of epidermal growth factor receptor mutation and anaplastic lymphoma kinase translocation in non-small cell lung cancer: do we know the treatment sequence? Cancer Res Stat Treat. 2019;2(1):119–120.
- Sweis RF, Thomas S, Bank B, et al. Concurrent EGFR mutation and ALK translocation in non-small cell lung cancer. Cureus. 2016;8(2):e513.
- Lou NN, Zhang XC, Chen HJ, et al. Clinical outcomes of advanced non-small-cell lung cancer patients with EGFR mutation, ALK rearrangement and EGFR/ALK co-alterations. Oncotarget. 2016;7(40):65185–65195.
- Shin HJ, Kho BG, Kim MS, et al. Co-alteration of EGFR mutation and ALK rearrangement in non-small cell lung cancer: case series. Medicine (Baltimore). 2019;98(9):e14699.
- Lo Russo G, Imbimbo M, Corrao G, et al. Concomitant EML4-ALK rearrangement and EGFR mutation in non-small cell lung cancer patients: a literature review of 100 cases. Oncotarget. 2017;8(35):59889–59900.
- Liu J, Mu Z, Liu L, et al. Frequency, clinical features and differential response to therapy of concurrent ALK/EGFR alterations in Chinese lung cancer patients. DDDT. 2019;13:1809–1817.
- Behel V, Noronha V, Patil V, et al. Molecular tumor board–guided treatment of non-small-cell lung cancer with dual driver (ALK and EGFR) alterations. Cancer Res Stat Treat. 2022;5(2):312–316.
- Akl EA, Briel M, You JJ, et al. Potential impact on estimated treatment effects of information lost to follow-up in randomised controlled trials (LOST-IT): systematic review. BMJ. 2012;344:e2809.
- Chakraborty S. A step-wise guide to performing survival analysis. Cancer Res Stat Treat. 2018;1(1):41–45.
- Dessai S, Simha V, Patil V. Stepwise cox regression analysis in SPSS. Cancer Res Stat Treat. 2018;1(2):167–170.
- Dessai S, Patil V. Testing and interpreting assumptions of COX regression analysis. Cancer Res Stat Treat. 2019;2(1):108–111.
- Pao W, Hutchinson KE. Chipping away at the lung cancer genome. Nat Med. 2012;18(3):349–351.
- Zhao Y, Wang S, Yang Z, et al. Co-occurring potentially actionable oncogenic drivers in non-small cell lung cancer. Front Oncol. 2021;11:665484.
- Patil VM, Noronha V, Joshi A, et al. Phase III study of gefitinib or pemetrexed with carboplatin in EGFR-mutated advanced lung adenocarcinoma. ESMO Open. 2017;2(1):e000168.
- Noronha V, Patil VM, Joshi A, et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J Clin Oncol. 2020;38(2):124–136.
- Bondili SK, Nandhana R, Noronha V, et al. Resistance mechanisms to epidermal growth factor receptor inhibitors in non-small cell lung cancer. Cancer Res Stat Treat. 2020;3(4):801–807.
- Kapoor A, Noronha V, Shetty O, et al. Molecular tumor board: case 2 – evolution of resistance in anaplastic lymphoma kinase driven non-small-cell lung carcinoma. Cancer Res Stat Treat. 2020;3(1):89–92.
- Zanwar S, Noronha V, Joshi A, et al. Efficacy of crizotinib in ALK mutant non-small cell lung cancers that are positive by IHC but negative by FISH compared to FISH positive cases. Indian J Cancer. 2017;54(4):678–680.
- Noronha V, Ramaswamy A, Patil VM, et al. ALK positive lung cancer: clinical profile, practice and outcomes in a developing country. PLoS ONE. 2016;11(9):e0160752.
- Pandey A, Rani M, Chandra N, et al. Impact of the coronavirus disease 2019 pandemic on cancer care delivery: a single-center retrospective study. Cancer Res Stat Treat. 2020;3(4):683–691.
- Pande P, Sharma P, Goyal D, et al. COVID-19: a review of the ongoing pandemic. Cancer Res Stat Treat. 2020;3(2):221–232.
- Noronha V, Shah D, Mokal S, et al. Deviation from standard cancer treatment during the first wave of the COVID-19 pandemic in India: a cross-sectional study. Cancer Res Stat Treat. 2022;5(2):212–219.
- Dettori JR. Loss to follow-up. Evid Based Spine Care J. 2011;2(1):7–10.
