 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Introduction
The presence of preoperative systemic inflammatory response (SIR) is an established negative prognostic factor for patients diagnosed with colorectal cancer (CRC). C-reactive protein (CRP) is known to be implicated in detrimental immune responses. The biological differences between right-sided and left-sided CRC are gaining increasing attention. Our aim was to analyse the prognostic value of CRP and explore the association between tumour location and SIR.
Material and methods
A total of 2059 patients treated for stage I–III CRC, identified from the prospectively sampled ScotScan Collaborative dataset, were included. The clinical and prognostic value of five CRP levels (<10/11–30/31–60/61–100/>100 mg/l) were examined. Additionally, the relationship between SIR and tumour location was explored.
Results
Increasing levels of CRP were associated with impaired overall and cancer-specific outcome. Presence of SIR was independently associated with right-sided tumour location (p<0.001). However, the impact of SIR on cancer-specific survival (CSS) was greater for left-sided tumour location, even when adjusted for other clinicopathological factors.
Conclusions
This study confirms CRP as a routinely available, valid, and clinically relevant strong prognostic marker of SIR in CRC patients. Right-sided tumours were more often associated with SIR, but the prognostic impact was stronger in left-sided tumours.
Introduction
Colorectal cancer (CRC) is the third most common type of cancer for men and women combined, and the second leading cause of cancer death worldwide [Citation1]. Different factors of cancer cells, tumour microenvironment, and host responses, contribute to diverging outcomes.
The presence of preoperative systemic inflammatory response (SIR) is an established negative prognostic factor for patients diagnosed with CRC of all stages [Citation2–4]. The modified Glasgow Prognostic Score (mGPS) has for two decades been recognised, and repeatedly validated, as to have independent prognostic value in cancer [Citation5,Citation6]. The introduction of the mGPS pioneered the presently widespread notion of the prognostic importance of SIR in cancer patients, and in CRC in particular. Accordingly, numerous different markers and scoring systems of inflammation have been proposed [Citation7,Citation8]. Many of the proposed markers may, however, be too complex to integrate into the everyday life in the oncology clinics. This is unfortunate considering the useful biological and clinical implication of SIR. The acute phase protein C-reactive protein (CRP), is a cheap and widely available marker, known to be implicated in detrimental immune responses [Citation9,Citation10], and previous studies have stated CRP alone, to be an adequate inflammation-based prognostic marker in CRC [Citation3,Citation11,Citation12].
An increasing amount of data emphasises that the large bowel can no longer be regarded as one entity, and primary tumour location as a stratification factor has been proposed [Citation13]. There are well-known differences between the right and left side of the colon. For cancer, these include differences in presentation, molecular subtypes, and oncogenic pathways [Citation14]. Sidedness is being recognised as a predictive marker with regards to treatment with targeted drugs [Citation15,Citation16], in addition to being a prognostic marker for patients with metastatic CRC [Citation16,Citation17]. Previous studies have suggested an association between systemic inflammation and right-sided primary tumour location [Citation18], however, the data are limited.
Given the strong correlation between presence of SIR and oncologic outcome, our aim was to analyse the prognostic value of CRP, by using one of the largest prospective cohorts of patients undergoing curative surgery for CRC stage I–III; the ScotScan collaborative dataset [2]. Furthermore, based on the increasing body of evidence demonstrating important differences between right and left colon, we explored the relationship between systemic inflammation and primary tumour location.
Material and methods
The ScotScan cohort
The ScotScan Collaborative dataset consists of data collected from a prospectively maintained database of patients diagnosed with CRC and treated at Glasgow Royal Infirmary, Scotland and Sørlandet Hospital, Norway [Citation2]. For this study, patients who underwent surgical treatment for stage I–III CRC with curative intent between 1997 and 2017 were included. Patients who underwent neoadjuvant chemoradiotherapy were excluded. Based on previous findings, patients were grouped into five CRP categories (<10, 11–30, 31–60, 61–100 and >100 mg/l) [Citation3,Citation11,Citation12]. Primary tumour location was classified as right (from caecum to distal traverse) and left (distal from the splenic flexure) including or excluding rectum as further specified for different analyses.
Serum CRP and albumin were measured within 30 days prior to resection for elective surgery, and on the day of hospital admission for emergency surgery. The mGPS was calculated as previously described [Citation5].
Patients received adjuvant chemotherapy consistent with national contemporary guidelines. Pathological staging differed between the two countries. In Norway staging was performed using TNM 5th edition until 2009, 7th edition until 2017 and later the 8th edition, whereas in Scotland the TNM 5th edition was used throughout the time period, in accordance with contemporary national guidelines [Citation19].
According to national guidelines, patients were routinely followed up for 5 years and due to uncertainties regarding cause of death after 5 years, survival analyses were performed using 5-year follow-up. Overall survival (OS) and cancer-specific survival (CSS) were measured from date of surgery until date of death. Cause and date of death were confirmed using hospital records in both countries, and additionally the National Death Registry in Norway.
Date of last recorded follow-up was on 1st January 2020 in Norway and on 30th June 2019 for patients from Scotland.
Statistics
All continuous data were categorised into groups. Clinicopathological characteristics and CRP categories were examined using analysis. The relationships between clinicopathological characteristics and tumour sidedness were analysed using binary logistic regression to calculate odds ratio (OR) and 95% confidence interval (CI).
The relationships between clinicopathological characteristics and OS and CSS were examined using Cox regression to calculate hazard ratio (HR) and 95% CI. In multivariate analysis, both in logistic and Cox regression, a backwards conditional model was conducted, using variables with p < 0.05 in univariate analysis. Five-year overall and cancer-specific survival were analysed by log-rank test to compare survival between groups and visualised by Kaplan–Meier curves.
To account for differences in treatment over the period studied, year of surgery was divided into quartiles and entered as a variable into all multivariate models.
A p-value <0.05 was considered statistically significant. All analyses were performed using SPSS version 28.0 for Mac (IBM Corp. SPSS 2020, Armonk NY, USA).
Results
Clinicopathological characteristics
The clinicopathological characteristics of 2059 patients of the ScotScan cohort who underwent resection of stage I–III CRC and without receiving any neoadjuvant treatment, are displayed in , according to preoperative CRP values. Median age was 72 years (min–max, 21–98 years). Median CRP value was 6.0 mg/l (75th percentile 17 mg/l). 66.4% of the patients had a preoperative CRP value of <10 mg/l, 26.5% had CRP value between 11–60 mg/l and 7.0% had CRP value >60 mg/l. Emergency presentation accounted for 10% of the patients. 21.3% of the patients received systemic adjuvant therapy. Right-sided primary tumour location was observed in 44.2% of the patients. Among patients with normal CRP (<10 mg/l), the majority (60.5%) had their primary tumour located on the left side of the colon. Follow-up was available for all patients with a median of 5.3 years (64 months, min–max, 0–274 months).
Table 1. Clinicopathological characteristics and associations to CRP values.
Comparison of prognostic value in OS and CSS of CRP and mGPS
These data are displayed in . Both SIR markers showed a statistically significant increased risk of event, and thus poorer survival, with elevated levels. For the clinical ease of usage, further analyses in this study were performed by using CRP cut-offs.
Table 2. Prognostic information regarding overall and cancer-specific survival for patients undergoing surgery for colorectal cancer stage I–III according to CRP and mGPS.
Relationship between clinicopathological characteristics and tumour sidedness
These data are displayed in , with left side including rectum. The clinicopathological characteristics were chosen based on clinical relevance with respect to prognosis and/or treatment. On univariate binary logistic regression analysis, advancing age, female sex, higher ASA score, later year of surgery quartile, advanced TNM stage, poor differentiation and elevated CRP levels were all associated with right-sided tumour location. Age (OR 1.25, 95% CI 1.10–1.42), year of surgery quartile (OR 1.15, 95% CI 1.05–1.25), and differentiation (OR 3.67, 95% CI 2.64–5.11) were all independently associated with right-sided tumour location in multivariate analyses. All CRP levels were independently associated with right-sided tumour location in an increasing manner (OR 1.64, 95% CI 1.25–2.16, OR 1.67, 95% CI 1.15–2.43, OR 1.88, 95% CI 1.07–3.28, respectively), until CRP reached > 100 mg/l (OR 1.33, 95% CI 0.76–2.33).
Table 3. Relationship between clinicopathological characteristics and tumour sidedness with left side as reference, including rectum.
Factors associated with 5-year CSS according to primary tumour location
Rectal cancer is often associated with other treatment regimens and modalities compared to colon cancer, in addition to proposed differences in biology [Citation20]. Therefore, the occurrence and impact of SIR were analysed separately for rectal cancer. The relationship between clinicopathological factors and CSS according to primary tumour location is shown in . The relationship between clinicopathological factors and OS is displayed in Supplementary Table 2. In univariate analyses, increasing CRP levels were associated with an increased HR for both right-sided and left-sided tumour location. When adjusting for other clinicopathological factors, however, the CRP levels remained significant only for primary left-sided tumours. For rectal primary tumours, the CRP level was not associated with CSS. Only 13 out of 444 (2.9%) patients with rectal cancer, exhibited a CRP value >60 mg/l.
Table 4. Relationship between clinicopathological factors and cancer-specific survival according to primary tumour location.
Relationship between CRP values and 5-year survival according to right- or left-sided primary tumour location
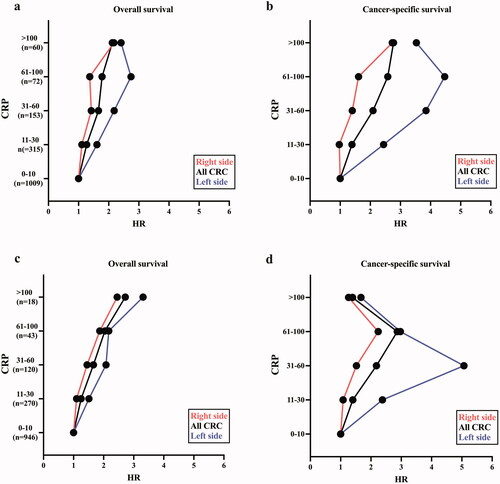
These data are depicted in ). As the presence of SIR seemed to be less pronounced and less relevant in rectal tumours, and due to abovementioned factors of differences in biology and treatment regiments, these analyses were performed excluding patients with primary rectal cancer. All HR values are adjusted to the clinicopathological factors presented above. When analysing the whole cohort (n = 1609), HR for both OS and CSS increased as the CRP level increased (). A similar ascend was observed for both right- and left-sided tumour location, however with higher HR for left-sided primary tumours. When excluding patients undergoing emergency operations and postoperative mortality within 30 days, a gradually increase in HR was observed for both right- and left-sided tumour patients (). Also, when excluding emergency surgery and postoperative death, an impaired CSS is observed in patients with an elevated CRP level. Most significant for patients with primary tumour on the left side, until CRP reaches > 60 mg/l (). All HR-, CI-, and p-values are shown in Supplementary Table 3(a–d). Clinicopathological characteristics of the patient cohort when excluding emergency surgery and postoperative mortality are shown in Supplementary Table 1. Log-rank tests and Kaplan–Meier plots for the whole cohort according to CRP levels are shown in Supplementary Figure 1.
Figure 1. (a–d) Relationship between C-reactive protein (CRP) values (in mg/l) and survival for patients undergoing curative treatment for colorectal cancer (CRC) according to tumour sidedness, with all patients shown in black, right side shown in red and left side (excluding rectum) shown in blue. The number of patients at each CRP level is noted as n below the respected level. All HRs are adjusted to clinicopathological factors (age, sex, ASA score, year of surgery, TNM-stage, and differentiation). All HR-, CI-, and p-values are shown in Supplementary Table 3(a–d). Clinicopathological characteristics of the patient cohort when excluding emergency surgery and postoperative mortality are shown in Supplementary Table 1. (a) The relationship between CRP level and OS according to tumour location. (b) The relationship between CRP level and CSS according to tumour location. (c) The relationship between CRP level and OS according to tumour location excluding patients undergoing emergency surgery (n = 206) and postoperative death (within 30 days of surgery, n = 40). (d) The relationship between CRP level and CSS according to tumour location excluding patients undergoing emergency surgery and postoperative death.
Discussion
By utilising one of the largest prospective datasets of resectable stage I–III CRC to date, the present work confirmed CRP as a valid, feasible and clinically simple-to-use strong prognostic marker of SIR. This study further revealed a strong association between the presence of SIR and right-sided primary tumour location, even when adjusted for other clinicopathological factors. Yet, once present on the left side of the large bowel, SIR exerts a much greater impact on CSS compared to right-sided tumour location. Furthermore, CRP values >60 mg/ml in patients with stage I–III CRC should lead to the suspicion of other causes than cancer-induced SIR.
This study adds to previous data showing that increasing levels of CRP are associated with increasingly worse OS and CSS, however, with a significant difference between right and left side for the latter. Even when adjusting for acute conditions that might have caused CRP to be elevated, by excluding patients with emergency surgery and postoperative mortality within 30 days, the tendency was even greater.
We were surprised to observe the decrease in HR for patients with left-sided primary tumour location when CRP reached >60 mg/l. Our data allowed us to investigate the patients from the Norwegian cohort, with left-sided primary tumour location (including rectum), CRP >60 mg/l, undergoing elective surgery and survived 30 days after surgery (n = 11). In eight out of eleven patients, we found evidence of another condition able to cause an elevated CRP (i.e., subclinical tumour perforation, abscess formation, and concomitant infection). This indicates that CRP values > 60 mg/l may represent an infection or other complications, rather than a cancer-associated SIR. Despite the limitation of a small number of patients and loss of statistical significance, we report this finding due to the clinical relevance and possibly useful information for clinicians in making decisions concerning cancer patients, especially in evaluating the possibility of concomitant systemic or local infection, subclinical tumour perforation, and/or bowel obstruction. It is also important to consider the CRP kinetics in this setting. When the CRP level does indicate a non-cancer-associated immune response, this will most likely also be reflected in a rapid rise in CRP, whereas a tumour-associated detrimental SIR presumably will have a more gradual course of ascent.
This study has several strengths. The ScotScan Collaborative dataset is one of the largest dataset of patients operated and treated for stage I–III CRC to date [Citation2]. The data capture is prospective in nature, established with the intention of investigating the role of SIR in CRC. Our research confirmed that right-sided tumour location was independently associated with advanced age and poor differentiation [Citation21,Citation22].
In the past recent years, an increasing number of markers of SIR have been proposed. They are often based on combinations of cells and/or proteins and assembled as combinations in form of ratios or scores, e.g., neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio, lymphocyte to monocyte ratio, CRP to albumin ratio, lymphocyte to CRP ratio, ratio of albumin to fibrinogen, ratio of fibrinogen to albumin, neutrophil-platelet score, lymphocyte-monocyte score, and ratio of NLR to prealbumin [Citation7,Citation8,Citation23,Citation24]. Unfortunately, our data does not allow us to comprehensively compare CRP to these other proposed prognostic markers of inflammation. Nevertheless, the mGPS was one of the first markers of SIR, demonstrating the significance of the presence of systemic inflammation in cancer [Citation25–27] and has over the years been unequivocally demonstrated in almost all types of cancer [Citation5,Citation6]. An abundance of literature has shown mGPS to be equivalent to other combined markers in predicting survival [Citation5,Citation6,Citation23]. Thus, by demonstrating CRP’s comparable prognostic value to mGPS, we assume CRP’s adequacy to other markers of SIR.
The choice of CRP threshold values into five categories is based on previous used cut-off values of CRP in colon cancer [Citation3,Citation11,Citation12]. The level of albumin gives highly valuable information regarding the patient’s present condition. However, hypoalbuminemia in patient populations with less inflammation is observed infrequently [Citation2,Citation28]. The different CRP levels may therefore differentiate further and provide important information regarding survival for patients with a normal albumin level. In addition, they follow a pragmatic approach and are easy to integrate into the clinic. Similar thresholds have been proposed by others [Citation29], further emphasising the increased risk of mortality with increasing levels of CRP.
Despite established biological differences between the right and left colon, classification according to tumour sidedness is controversial and may represent the different sections of the large bowel incompletely. Studies have suggested that differences along the colon may represent a continuum, rather than clear cut sections [Citation14,Citation21]. Additionally, the same studies showed the transverse colon to be more similar to the left-sided colon in tumour mutational profiles [Citation14] and a distinct tumour biology in rectal cancers [Citation21], which may call for a more precise description based on tumour site, not sidedness. Unfortunately, our data do not allow us to evaluate mutational status or molecular subtypes, characteristics known to be associated with tumour location [Citation30,Citation31]. Nevertheless, our study fuels the proposition of important biological distinctions between the different sections of the large intestine and provides important insight into further research on the significance of tumour location in CRC.
The scientific awareness towards the composition of immune cells in the tumour microenvironment has during the recent years escalated. The tumour microenvironment comprises a high diversity of different immune cells with the abilities to acquire an anti- or pro-tumour activity [Citation32], with the release of cytokines/chemokines able to reach beyond the local environment and evoke systemic responses. The presence or absence of SIR, assessed by the CRP level, may therefore be associated with the immune cell composition of the tumour microenvironment. Indeed, Køstner et al. recently found that systemic inflammation, seen by an elevated level of CRP, correlated with a tumour microenvironment dominated by neutrophils and macrophages [Citation33].
Correspondingly, the prognostic value of CRP raises the question of which role CRP itself may exert within the tumour microenvironment. Research has for the last years revealed CRP as more than a circulatory protein whose levels increase during illness, and the biological roles of CRP are gradually becoming apparent [Citation9,Citation34–36]. In a recent study by Yoshida et al., CRP was found to have a profound suppressive effect on adaptive immunity in peripheral blood in melanoma patients [Citation10], with confirmed shorter OS in patients with elevated CRP in the same patient group [Citation37]. Taken together, these finding suggest that CRP represents more than a passive marker, and rather indicates CRP as an active participant in the process of ongoing detrimental immune responses.
In conclusion, CRP is a valid, readily available, and biological relevant strong prognostic marker of SIR in CRC. Even though more often present in right-sided tumour cases, SIR exerts a greater impact on survival once present in patients with left-sided CRC. The level of CRP may additionally be an indicator for clinicians when to consider the presence of a non-cancer-associated immune response. This work accentuates the indication of including CRP assessment in CRC patients, both as part of routine clinical practice and in future trials.
Ethical approval
Local Institutional approval was granted for use of data from both Glasgow Royal Infirmary (West Scotland Ethics Committee) and Centre for Cancer Treatment Sørlandet Hospital. The study was performed in accordance with the Declaration of Helsinki.
Author contributions
A.J.F wrote the original draft of the article, as well as revised/edited and approved the final article, assembled data, and performed statistical analyses and interpretation. S.M. participated in revision and editing of the original draft of the article, as well as interpretation of statistical analyses. A.H.R. performed revision and editing of the first draft of the article. D.C.M. designed the study, assembled, and interpreted data, as well as revised and edited the first draft of the article. J.H.P. designed the study, participated in revision and editing of the first draft of the article, in addition to assembling data. C.K. designed the study, collected and interpreted data, as well as revised and edited the first draft of the article. All authors revised and authorised the final submitted draft of the manuscript.
Supplemental Material
Download MS Word (236.3 KB)Acknowledgements
We thank Bente Mirjam Christensen for her dedicated and extensive work in maintaining and preserving the Sørlandet Hospital Colorectal Cancer Dataset.
Disclosure statement
A.J.F. has received honoraria from Novartis. No potential conflict of interest was reported by the author(s).
Data availability statement
Anonymised data for this study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249.
- Park JH, Fuglestad AJ, Køstner AH, et al. Systemic inflammation and outcome in 2295 patients with stage I-III colorectal cancer from Scotland and Norway: first results from the ScotScan Colorectal Cancer Group. Ann Surg Oncol. 2020;27(8):2784–2794.
- Thomsen M, Kersten C, Sorbye H, et al. Interleukin-6 and C-reactive protein as prognostic biomarkers in metastatic colorectal cancer. Oncotarget. 2016;7(46):75013–75022.
- Tuomisto AE, Mäkinen MJ, Väyrynen JP. Systemic inflammation in colorectal cancer: underlying factors, effects, and prognostic significance. World J Gastroenterol. 2019;25(31):4383–4404.
- McMillan DC. The systemic inflammation-based glasgow prognostic score: a decade of experience in patients with cancer. Cancer Treat Rev. 2013;39(5):534–540.
- Dolan RD, Lim J, McSorley ST, et al. The role of the systemic inflammatory response in predicting outcomes in patients with operable cancer: systematic review and meta-analysis. Sci Rep. 2017;7(1):16717.
- Ying HQ, Liao YC, Sun F, et al. The role of Cancer-Elicited inflammatory biomarkers in predicting early recurrence within stage II-III colorectal cancer patients after curable resection. J Inflamm Res. 2021;14:115–129.
- Yasui K, Shida D, Nakamura Y, et al. Postoperative, but not preoperative, inflammation-based prognostic markers are prognostic factors in stage III colorectal cancer patients. Mar. Br J Cancer. 2021;124(5):933–941.
- McFadyen JD, Kiefer J, Braig D, et al. Dissociation of C-reactive protein localizes and amplifies inflammation: evidence for a direct biological role of C-reactive protein and its conformational changes. Front Immunol. 2018;9:1351–1351.
- Yoshida T, Ichikawa J, Giuroiu I, et al. C reactive protein impairs adaptive immunity in immune cells of patients with melanoma. J Immunother Cancer. 2020;8(1):e000234.
- Kersten C, Louhimo J, Ålgars A, et al. Increased C-reactive protein implies a poorer stage-specific prognosis in colon cancer. Acta Oncol. 2013;52(8):1691–1698.
- Køstner AH, Kersten C, Löwenmark T, et al. The prognostic role of systemic inflammation in patients undergoing resection of colorectal liver metastases: C-reactive protein (CRP) is a strong negative prognostic biomarker. J Surg Oncol. 2016;114(7):895–899.
- Petrelli F, Tomasello G, Borgonovo K, et al. Prognostic survival associated with left-sided vs right-sided colon cancer: a systematic review and meta-analysis. JAMA Oncol. 2017;3(2):211–219.
- Loree JM, Pereira AAL, Lam M, et al. Classifying colorectal cancer by tumor location rather than sidedness highlights a continuum in mutation profiles and consensus molecular subtypes. Clin Cancer Res. 2018;24(5):1062–1072.
- Boeckx N, Koukakis R, Op de Beeck K, et al. Primary tumor sidedness has an impact on prognosis and treatment outcome in metastatic colorectal cancer: results from two randomized first-line panitumumab studies. Ann Oncol. 2017;28(8):1862–1868.
- Tejpar S, Stintzing S, Ciardiello F, et al. Prognostic and predictive relevance of primary tumor location in patients with RAS wild-type metastatic colorectal cancer: retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA Oncol. 2017;3(2):194–201.
- Holch JW, Ricard I, Stintzing S, et al. The relevance of primary tumour location in patients with metastatic colorectal cancer: a meta-analysis of first-line clinical trials. Eur J Cancer. 2017;70:87–98.
- Patel M, McSorley ST, Park JH, et al. The relationship between right-sided tumour location, tumour microenvironment, systemic inflammation, adjuvant therapy and survival in patients undergoing surgery for Colon and rectal cancer. Br J Cancer. 2018;118(5):705–712.
- Loughrey M, Quirke P, Shepherd N. Dataset for colorectal cancer histopathology reports. 3rd ed. London: The Royal College of Pathologist; 2014.
- Paschke S, Jafarov S, Staib L, et al. Are Colon and rectal cancer two different tumor entities? A proposal to abandon the term colorectal cancer. Int J Mol Sci. 2018;19(9):2577.
- Salem ME, Weinberg BA, Xiu J, et al. Comparative molecular analyses of left-sided colon, right-sided colon, and rectal cancers. Oncotarget. 2017;8(49):86356–86368.
- Peng J, Li C, Wang F, et al. Right- and left-sided stage III Colon cancers present different prognostic outcomes of oxaliplatin-based adjuvant chemotherapy after curative resection. CMAR. 2018;10:2095–2103.
- Dolan RD, McSorley ST, Park JH, et al. The prognostic value of systemic inflammation in patients undergoing surgery for Colon cancer: comparison of composite ratios and cumulative scores. Br J Cancer. 2018;119(1):40–51.
- Colloca GA, Venturino A, Guarneri D. Second-generation inflammation-related scores are more effective than systemic inflammation ratios in predicting prognosis of patients with unresectable or metastatic pancreatic cancer receiving cytotoxic chemotherapy. Med Oncol. 2018;35(12):158.
- McMillan DC, Elahi MM, Sattar N, et al. Measurement of the systemic inflammatory response predicts cancer-specific and non-cancer survival in patients with cancer. Nutr Cancer. 2001;41(1–2):64–69.
- Scott HR, McMillan DC, Forrest LM, et al. The systemic inflammatory response, weight loss, performance status and survival in patients with inoperable non-small cell lung cancer. Br J Cancer. 2002;87(3):264–267.
- Forrest LM, McMillan DC, McArdle CS, et al. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br J Cancer. 2003;89(6):1028–1030.
- Park JH, Ishizuka M, McSorley ST, et al. Staging the tumor and staging the host: a two Centre, two country comparison of systemic inflammatory responses of patients undergoing resection of primary operable colorectal cancer. Am J Surg. 2018;216(3):458–464.
- Marsik C, Kazemi-Shirazi L, Schickbauer T, et al. C-reactive protein and all-cause mortality in a large hospital-based cohort. Clin Chem. 2008;54(2):343–349.
- Mooi JK, Wirapati P, Asher R, et al. The prognostic impact of consensus molecular subtypes (CMS) and its predictive effects for bevacizumab benefit in metastatic colorectal cancer: molecular analysis of the AGITG MAX clinical trial. Ann Oncol. 2018;29(11):2240–2246.
- Guinney J, Dienstmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21(11):1350–1356.
- Bruni D, Angell HK, Galon J. The immune contexture and immunoscore in cancer prognosis and therapeutic efficacy. Nat Rev Cancer. 2020;20(11):662–680.
- Køstner AH, Nielsen PS, Georgsen JB, et al. Systemic inflammation associates with a myeloid inflamed tumor microenvironment in primary resected colon cancer—may cold tumors simply be too hot? Front Immunol. 2021;12:716342.
- Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754.
- Rajab IM, Hart PC, Potempa LA. How C-reactive protein structural isoforms with distinctive bioactivities affect disease progression. Front Immunol. 2020;11:2126.
- Hart PC, Rajab IM, Alebraheem M, et al. C-Reactive protein and cancer-diagnostic and therapeutic insights. Front Immunol. 2020;11:595835.
- Laino AS, Woods D, Vassallo M, et al. Serum interleukin-6 and C-reactive protein are associated with survival in melanoma patients receiving immune checkpoint inhibition. J Immunother Cancer. 2020;8(1):e000842.
