?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
The impact of the tumour volume or size on achieving clinical complete response (cCR) after radio(chemo)therapy is poorly understood.
Materials and methods
A literature search was performed to gather data on the predictive value of baseline tumour volume or size in achieving cCR.
Results
In total, nine reports were identified. In two of three studies evaluating the baseline tumour volumetry, the tumour volume was the most powerful predictor for cCR. In four of six studies evaluating baseline tumour size without volumetry, tumour dimension was significantly associated with cCR, in one study reached borderline significance and in one report was insignificant. In three of four studies where a multivariable analysis was performed, the cT category did not show an independent predictive value for cCR. Because the tumour shape is often (semi)annular, its circumferential rectal extent along with the tumour length probably impact the tumour volume most, and thus, could be considered an acceptable alternative for time-consuming volumetry.
Conclusions
Our review suggests that baseline tumour volume (or alternatively, tumour length along with its circumferential rectal extent) is the most relevant clinical predictor of cCR. Therefore, we postulate assessing and reporting these parameters in studies on the watch-and-wait strategy.
Keywords:
Background
The watch-and-wait strategy in patients with clinical complete response (cCR) after radio(chemo)therapy is already routine management in many institutions worldwide. The rate of cCR reported in the literature has varied between 5 and 88% [Citation1–12]. The differences in radio(chemo)therapy doses [Citation1–3,Citation7,Citation9], TN-categories [Citation3–5,Citation11] and time elapsed between radio(chemo)therapy and tumour response evaluation [Citation13] can explain this wide variation. However, even though these three parameters have been similar, cCR rates have still varied considerably across studies. Thus, there is a need to look for additional predictors that can inform individual patients of their chances for the watch-and-wait strategy and enable comparison of the efficacy of different radio(chemo)therapy methods for achieving cCR.
The predictive value of tumour volume or size in watch-and-wait strategies is poorly understood, thus needing further exploration. The main aim of this article is to conduct a literature search to gather all published data on the predictive value of tumour volume or size in achieving cCR.
Materials and methods
Review of the literature
Reports in English showing the rate of initial cCR or long-term sustained cCR in relation to tumour volume or size in patients receiving radio(chemo)therapy were eligible for our review. cCR was defined as whitening of the mucosa, telangiectasia with mucosal integrity without any superficial ulcer, irregularity or palpable nodule or significant stenosis. We did not include articles evaluating the occurrence of pathological complete response (pCR) because of the discordance between cCR and pCR, especially in large tumours [Citation4,Citation14]. This discordance is caused by two phenomena. Firstly, after radiotherapy, the residual macroscopic exophytic abnormalities or deep ulcers that do not contain cancer on microscopic assessment (pCR) often mimic cancer clinically. For example, Smith et al. [Citation14] compared the macroscopic rectal mucosal appearance in postoperative specimen with the final pathological stage. Of the patients with pCR, 74% had a residual mucosal abnormality that precluded the diagnosis of cCR. Secondly, subclinical cancer is often found in patients with cCR. For example, in the above publication, 27% of patients with cCR still had subclinical residual disease as assessed by microscopy.
One of the authors (KB) searched the PubMed database for articles published up to August 2022. Articles were screened according to their titles and abstracts using the following search terms: ‘rectal cancer’ OR ‘rectal adenocarcinoma’ AND ‘non-operative management’ OR ‘clinical complete response’ OR ‘non-operative treatment’ OR ‘organ preservation’ AND ‘watch-and-wait’. This search was supplemented by forward and backward citation tracking from included studies and by searching abstracts from ASCO, ESTRO and ASTRO 2019–2022 meetings. There was no formal protocol for this review.
Results
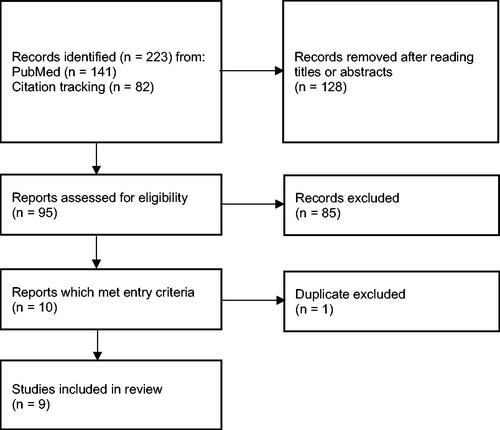
In total, 223 records were identified through online PubMed searching and citation tracking. Of these, nine articles met the eligibility criteria (, ). Seven studies looked at initial cCR only, one study looked at sustained cCR only, and one study looked at both initial and sustained cCR. The marked heterogeneity in patients’ characteristics and interventions make impossible performing quantitative analysis through meta-analysis. Thus, narrative summary was only performed.
Table 1. The design and outcomes of the studies evaluating the association of baseline tumour volume or size with clinical complete response.
Three studies explored the association between tumour volume and initial or sustained cCR. In the largest study by Jankowski et al. [Citation4], primary tumour was contoured and next tumour volume was automatically measured by a radiotherapy planning workstation. In the multivariable analysis, only the increasing tumour volume (or alternatively, the increasing tumour length and proportion of circumferential extent within the rectal wall) and the cN + category were statistically significant negative predictors for initial cCR. No cCR was found among the 27 patients with a tumour volume larger than 120 cc. In the study by Benezery et al. [Citation3], tumour volume was calculated using 3-dimensional measurements and geometrical formulas. In the univariable analysis, the treatment chronology (external beam radiation first vs. brachytherapy first), and circumferential extent within the rectal wall were associated with initial cCR or near-cCR combined. Tumour volume reached borderline significance (p = 0.075). In the multivariable analysis, only brachytherapy first was significant positive predictor. In the study by Rijkmans et al. [Citation9], the tumour was delineated on baseline MRI to assess its volume. The tumour volume was the only significant predictor of initial cCR (p = 0.005). Of the patients with tumour volume less than 20 cc, 80% achieved cCR, as opposed to only 25% of those with a larger volume. No cCR was found among the 10 patients with a tumour volume larger than 25 cc. At significance level of p < 0.01, tumour thickness, proportion of circumferential extent within the rectal wall and the cN-category showed a trend (p-value between 0.01 and 0.1) for an association with cCR. For sustained cCR a trend was observed for baseline tumour volume (p = 0.02), circumferential involvement (p = 0.02) and thickness (p = 0.03).
Six studies evaluated cCR rate in relation to the primary tumour size measurements without volumetry. Yuval et al. [Citation15] reported an analysis of the predictors of sustained cCR, evaluated in the patients participating in the randomised phase II OPRA trial. In the multivariable analysis, three parameters were significantly (p < 0.05) associated with cCR: involvement of the mesorectal fascia, clinical N + stage and the presence of extramural vascular invasion. Tumour length reached borderline significance (p = 0.066). The association with cT category was not significant. In the study by Jimenez-Rodriguez et al. [Citation16], tumour length was the only parameter significantly associated with initial cCR, with a median of 3.8 cm in the patients achieving cCR and 4.8 cm in those with a persistent lesion (p = 0.003). Gerard et al. [Citation8] found three parameters significantly associated with initial cCR: the cT category, tumour diameter and its circumferential extent within the rectal wall. A tumour diameter measurement method was not provided. cCR was diagnosed in 13.8% of patients with a tumour diameter not exceeding 4 cm vs. 2.9% in those with a larger tumour (p = 0.017). For patients with a tumour involving less than half of the rectal circumference and for those with a larger circumferential extent within the rectal wall, initial cCR rates were 12.5 vs. 5.0%, respectively (p = 0.04). In a series reported by Habr-Gama et al. [Citation5], the tumour size was not associated with initial cCR. A tumour size measurement method was not provided. The mean tumour size in the cCR group was 36 vs. 38 mm in the nonresponder group (p = 0.23). In the study by Chin et al. [Citation11], tumour size (defined as the maximum diameter), mesorectal fascia involvement and cT category were significantly associated with initial cCR. The median tumour size in the cCR group was 4.0 cm vs. 5.2 cm in the nonresponder group (p = 0.002). In the propensity-score matched study by Mbanu et al. [Citation17], the multivariable analysis showed that tumour length and cT-category were significantly associated with initial cCR.
Discussion
Our literature review showed that of three studies evaluating the baseline tumour volumetry, in two the tumour volume was the most powerful predictor for cCR () [Citation4,Citation9]. In four of six studies evaluating tumour size without volumetry, tumour dimension was significantly associated with cCR, in one study reached borderline significance and in one report was insignificant () [Citation8,Citation11,Citation15–17]. The importance of tumour size has been highlighted in the recently presented OPERA randomised trial [Citation18]. At 3 years, organ preservation in tumours smaller than 3 cm was as high as 97% in the patients assigned to chemoradiation (45 Gy) combined with X-ray brachytherapy and 65% in those assigned to chemoradiation alone (54 Gy). It is unclear why in the publication by Jankowski et al. [Citation4] no cCR was found in the patients with tumour volume larger than 120 cc, whereas in the publication by Rijkmans et al. [Citation9] this cut-off point was only 25 cc. This discrepancy could be related to only 10 patients with tumour volume larger than 25 cc in the latter report.
These observations were confirmed in a study evaluating correlation between baseline tumour volumetry and pCR; multivariable analysis showed that tumour volume was the only predictor of pCR [Citation19]. In another study, the pCR prediction accuracy was 73% using a tumour volume cut-off of 15 cc [Citation20]. It is worth noting that a significant association between tumour volume or size and cCR was observed in most studies regardless of differences in tumour characteristics, treatment methods and time intervals elapsed between treatment and tumour response evaluation. Such association was found in patients receiving routine radiation(chemo)therapy [Citation4,Citation8,Citation17], in patients with small tumours receiving brachytherapy boost [Citation3,Citation9] and in those with advanced cancer receiving total neoadjuvant therapy by using induction or consolidation chemotherapy [Citation11,Citation15,Citation16] (). Recently published results of the OPRA randomised trial showed that sustained cCR was more often achieved with consolidation chemotherapy than with induction chemotherapy [Citation12]. The above findings are in line with well-known observations in cervical, hand and neck and breast cancers as well as in melanomas, showing that the tumour volume is the strongest predictor for local control when radiotherapy is being used as the only treatment [Citation21]. Increasing tumour size was significantly associated with higher risk of local failure in squamous cell carcinoma of anal cancer [Citation22]
In three of four studies where a multivariable analysis was performed, the cT category did not show an independent predictive value for cCR () [Citation3,Citation4,Citation15,Citation17]. Thus, assessing only the cT category is insufficient to determine the chance of cCR accurately. It is likely, that this is because the tumour volumes largely overlap between the cT categories (). Notably, tumour volumetry and the measurements of the tumour size are more reproducible than T-categorising [Citation19,Citation23]. The difficulty in distinguishing between cT2 and cT3a leads to overstaging [Citation23]. Subclassification of cT3 disease according to the version 5 of TNM may be relevant in achieving cCR [Citation24]. Probably the separation of cT2 and cT3a is not so important. On the other hand, distinguishing between cT2 and cT3 could be important when tumour volume is similar, especially in studies on radiation or chemotherapy dose escalation [Citation1,Citation25,Citation26]. It is likely that a less aggressive and more radio-curable phenotype is more common in cT2 than in cT3 tumours. For example, it was shown that radiation dose escalation and the addition of chemotherapy after chemoradiation led to an improvement in surgery-free survival in cT2 cancers [Citation25] but not in cT3 cancers [Citation26]. The type of cancer infiltration and the presence of central ulceration could be different between cT2 and cT3 lesions.
Figure 2. Tumour volume in relation to the cT category in 360 patients. This previously unpublished analysis was performed for the purpose of the present study using the database from a previous publication [Citation4] co-authored by two of the current authors. The box indicates the interquartile range. The thick horizontal line within the box indicates the median value. The vertical bar indicates the location of 95% measurements.
![Figure 2. Tumour volume in relation to the cT category in 360 patients. This previously unpublished analysis was performed for the purpose of the present study using the database from a previous publication [Citation4] co-authored by two of the current authors. The box indicates the interquartile range. The thick horizontal line within the box indicates the median value. The vertical bar indicates the location of 95% measurements.](/cms/asset/2f8d8c3c-ada1-4fa2-9e72-df7ae5df38ae/ionc_a_2122866_f0002_c.jpg)
There are three main methods of measuring tumour size as follows: (1) a 1-dimensional maximum diameter measurement; (2) 3-dimensional size measurements and the use of a geometric formula to calculate tumour volume; and (3) a measurement of the whole tumour volume by manually contouring the tumour boundaries on each section containing the tumour and using dedicated volume calculation software [Citation20]. The accuracy of the 1-dimensional and 3-dimensional measurements is hampered by the uncertainty regarding how a tumour should be measured on the axial MRI image – see Supplementary Figure 1. Using baseline MRI, interobserver agreement was good for the 1-dimensional and 3-dimensional measurements and excellent for the whole-volume measurements [Citation20]. Our review and the review evaluating pCR [Citation20] suggest that the whole volume measurements offer the best method for the prediction of complete response. In the future, developing accurate automated segmentation techniques could replace time-consuming manual contouring.
Could tumour length along with its circumferential extent within the rectal wall be considered an acceptable alternative for tumour volumetry?
The recent guidelines of the European Society of Gastrointestinal and Abdominal Radiology do not require tumour volume measurement on MR images. Instead, reporting the tumour length and its circumferential extent within the rectal wall is recommended [Citation23]. Thus, these measurements should be easily available. In addition, they can be validated by digital rectal examination and endoscopy. Thus, the question arises as to whether measurement of tumour length along with the proportion of circumferential extent within the rectal wall could be considered an acceptable alternative for tumour volumetry. The multivariable analysis performed in the largest study included in our review showed that of the primary tumour characteristics, only the tumour volume impacted cCR independently and significantly () [Citation4]. However, when the tumour volume was replaced by the tumour length and proportion of circumferential extent within the rectal wall, only these two primary tumour parameters showed independent statistically significant associations with cCR. Of five studies evaluating the association between tumour length and cCR, in three studies this association was significant in one was of borderline significance and in one was insignificant () [Citation4,Citation9,Citation15–17]. Of four studies evaluating the association between circumferential extent within the rectal wall and cCR, in three studies this association was significant and in one was of borderline significance () [Citation3,Citation4,Citation8,Citation9].
Because tumour shape is often (semi)annular, it is clear that its circumferential extent within the rectal wall must also substantially impact tumour volume. The actual width of the tumour should be measured as a curved (not straight) line along its circumferential extent within the rectal wall (Supplementary Figure 1). All of the above suggest that, for practical reasons, tumour length along with its circumferential extent within the rectal wall could be considered an acceptable alternative for time-consuming tumour volumetry.
Practical implications
cT-categorising, evaluation of mesorectal fascia threatening and involvement of the intersphincteric plane have been mostly developed to guide surgical treatment. By contrast, when non-operative management is considered, this review suggests that tumour volume becomes the most important factor. Thus, we suggest routine assessment and reporting of the baseline tumour volume or alternatively, tumour length (e.g. proportion of tumours with ≤3, >3–5 and >5 cm) along with the proportion of circumferential extent within the rectal wall (e.g. proportion of tumours involving ≤50%, >50% to <100% and 100% [circular]) in candidates for the watch-and-wait strategy. This would enable the informing of patients in shared decision-making of their individual chance for non-operative management, as well as an unbiased comparison of the efficacy of different treatment methods in achieving cCR. If the tumour volume is not considered, the true influence of the treatment method might be masked. Reporting data about ‘tumour diameter’ or ‘tumour size’ is insufficient without defining these terms. Finally, using tumour volume (or tumour length along with the proportion of circumferential extent) as a stratification factor in randomised studies on the watch-and-wait strategy would assure balanced patient allocation between arms.
Why does increasing tumour volume adversely affect the outcomes of organ-preserving treatment?
Increasing tumour volume negatively impacts on cCR and final local control for three reasons. (1) cCR is dependent on the number of clonogens requiring sterilisation and on tumour hypoxia, which increase with the tumour volume. This effect is augmented by two other phenomena. (2) Most of the sterilised (defined as the sum of sustained cCR and pCR in non-cCR patients) large-volume cancers do not have cCR because a persistent fibrous-only tumour or ulcer clinically mimics a viable cancer [Citation4,Citation14]. Of the patients with cancer sterilisation, a persistent tumour without cancer was found in 21% having a small baseline tumour (≤50% of the circumferential extent and length ≤4 cm), whereas this rate was as high as 77% in the patients having a large baseline tumour (circular cancers or with a length ≥7 cm) [Citation4]. (3) Of patients who achieve cCR and undergo a watch-and-wait strategy, those with a large cancer at baseline are at higher risk of regrowth than are those with a small cancer [Citation1,Citation27]. This is illustrated by a series of 74 patients treated with contact X-ray brachytherapy and chemoradiation, the majority of whom underwent a watch-and-wait strategy [Citation1]. The regrowth rate at 3 years was 8.5% in the group with a tumour size≤3 cm compared with 13.0% in the group with larger tumours (p = 0.04).
Categorising the tumour by the chance of achieving cCR
Three attempts were identified to categorise tumours according to their volume or dimensions at baseline in relation to watch-and-wait strategy outcomes. Benezery et al. [Citation3] reported excellent results in 23 patients with polypoid tumour less than 3 cm in the largest dimension treated with contact X-ray brachytherapy and chemoradiation; all achieved cCR and none had local recurrence. Rijkmans et al. [Citation9] in their series of 38 patients treated with external beam radiotherapy and iridium brachytherapy boost, observed sustained response at 2 years in 74% of the patients with a baseline tumour volume <20 cc compared with only 25% of those with larger cancers (p = 0.007). Considering the shape of the curve showing the association between tumour volume and cCR achieved after routine preoperative radio(chemo)therapy doses (), Jankowski et al. [Citation4] concluded that it is easier to determine a tumour size threshold for a very low chance of cCR than for a high chance. Circular tumour or tumour length ≥7 cm was proposed for such a threshold, which corresponded to cancer volumes above 45 cc in 75% of the patients. In this subgroup, cCR was achieved in only 3% of the patients receiving routine preoperative radiotherapy. In the most favourable subgroup, which consisted of patients having a tumour length ≤4 cm and ≤50% circumferential extent within the rectal wall (which corresponded to a volume below 23 cc in 75% of the patients), cCR was achieved in 34% of the patients.
Figure 3. Regression curve showing the association between tumour volume and the probability of clinical complete response (cCR) achieved after routine doses of preoperative radiotherapy (5 × 5 Gy with delayed surgery or 5 × 5 Gy + 6 weeks of consolidation chemotherapy or chemoradiation using 50 Gy, 2 Gy per fraction) in 360 patients. Reproduced from [Citation4] with permission from Elsevier.
![Figure 3. Regression curve showing the association between tumour volume and the probability of clinical complete response (cCR) achieved after routine doses of preoperative radiotherapy (5 × 5 Gy with delayed surgery or 5 × 5 Gy + 6 weeks of consolidation chemotherapy or chemoradiation using 50 Gy, 2 Gy per fraction) in 360 patients. Reproduced from [Citation4] with permission from Elsevier.](/cms/asset/1866c307-ce65-4e83-b201-204b028c17f5/ionc_a_2122866_f0003_b.jpg)
Limitations
Evaluation of predictive factors for cCR was not a primary endpoint in any of the prospective studies included in this review and was a secondary endpoint in only one. In this study, tumour volume was added post hoc and available in 85% of the patients [Citation4]. Our suggestion that tumour length along with its circumferential extent within the rectal wall could be used as an acceptable alternative for tumour volumetry was not validated. Therefore, this suggestion should be treated with caution. Our review is not based on a formal protocol and therefore may not be considered of high scientific value. The rate of long-term sustained cCR is more important endpoint than the rate of initial cCR. However, only in two of nine included reports, tumour volume or size was correlated with the sustained cCR. In seven remaining reports, the correlations were performed with the initial cCR. Nevertheless, we believe that our conclusions are acceptable because there is a high correlation between initial cCR and sustained cCR.
Conclusions
Our review suggests that the baseline tumour volume (or alternatively, tumour length along with its circumferential extent within the rectal wall) is the most relevant clinical predictor of cCR. Therefore, we postulate assessing and reporting these parameters in studies on watch-and-wait strategies.
Supplemental Material
Download MS Word (616.2 KB)Acknowledgements
The authors thank Dr Joanna Socha for her help in writing the manuscript. There was no supporting source for this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The database has been deposited in a repository and is available upon request from the corresponding author [KB].
References
- Gérard JP, Barbet N, Gal J, et al. Planned organ preservation for early T2-3 rectal adenocarcinoma: a French, multicentre study. Eur J Cancer. 2019;108:1–16.
- Appelt AL, Pløen J, Harling H, et al. High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: a prospective observational study. Lancet Oncol. 2015;16(8):919–927.
- Benezery K, Montagne L, Evesque L, et al. Clinical response assessment after contact X-Ray brachytherapy and chemoradiotherapy for organ preservation in rectal cancer T2-T3 M0: the time/dose factor influence. Clin Transl Radiat Oncol. 2020;24:92–98.
- Jankowski M, Pietrzak L, Rupiński M, Polish Colorectal Study Group, et al. Watch-and-wait strategy in rectal cancer: is there a tumour size limit? Results from two pooled prospective studies. Radiother Oncol. 2021;160:229–235.
- Habr-Gama A, Gama-Rodrigues J, São Julião GP, et al. Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: impact of salvage therapy on local disease control. Int J Radiat Oncol Biol Phys. 2014;88(4):822–828.
- Nahas SC, Rizkallah Nahas CS, Sparapan Marques CF, et al. Pathologic complete response in rectal cancer: Can We detect it? Lessons learned from a proposed randomized trial of watch-and-wait treatment of rectal cancer. Dis Colon Rectum. 2016;59(4):255–263.
- Garant A, Magnan S, Devic S, et al. Image guided adaptive endorectal brachytherapy in the nonoperative management of patients with rectal cancer. Int J Radiat Oncol Biol Phys. 2019;105(5):1005–1011.
- Gérard JP, Chamorey E, Gourgou-Bourgade S, et al. Clinical complete response (cCR) after neoadjuvant chemoradiotherapy and conservative treatment in rectal cancer. Findings from the ACCORD 12/PRODIGE 2 randomized trial. Radiother Oncol. 2015;115(2):246–252.
- Rijkmans EC, Marijnen CAM, van Triest B, et al. Predictive factors for response and toxicity after brachytherapy for rectal cancer; results from the HERBERT study. Radiother Oncol. 2019;133:176–182.
- van der Valk MJM, Hilling DE, Bastiaannet E, IWWD Consortium, et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the international watch & wait database (IWWD): an international multicentre registry study. Lancet. 2018;391(10139):2537–2545.
- Chin RI, Roy A, Pedersen KS, et al. Clinical complete response in patients with rectal adenocarcinoma treated with short-course radiation therapy and nonoperative management. Int J Radiat Oncol Biol Phys. 2022;112(3):715–725.
- Garcia-Aguilar J, Patil S, Gollub MJ, et al. Organ preservation in patients with rectal adenocarcinoma treated with total neoadjuvant therapy. J Clin Oncol. 2022;40(23):2546–2556.
- Habr-Gama A, São Julião GP, Fernandez LM, et al. Achieving a complete clinical response after neoadjuvant chemoradiation that does not require surgical resection: it may take longer than you think!. Dis Colon Rectum. 2019;62(7):802–808.
- Smith FM, Wiland H, Mace A, et al. Clinical criteria underestimate complete pathological response in rectal cancer treated with neoadjuvant chemoradiotherapy. Dis Colon Rectum. 2014;57(3):311–315.
- Yuval JB, Verheij FS, Lin ST, On behalf of the OPRA consortium, et al. Clinical and radiological predictors of organ preservation in patients with rectal cancer treated with total neoadjuvant therapy. J Clin Oncol. 2022;40(16_suppl):3619–3619.
- Jimenez-Rodriguez RM, Quezada-Diaz F, Hameed I, et al. Organ preservation in patients with rectal cancer treated with total neoadjuvant therapy. Dis Colon Rectum. 2021;64(12):1463–1470.
- Mbanu P, Osorio EV, Mistry H, et al. Clinico-pathological predictors of clinical complete response in rectal cancer. Cancer Treat Res Commun. 2022;31:100540.
- Gerard J-P, Barbet NN, Pacé-Loscos T, et al. Contact x-ray brachytherapy (papillon) in addition to chemoradiotherapy to improve organ preservation in early cT2-T3 rectal adenocarcinoma: the 3-year results of OPERA randomized trial (NCT02505750). J Clin Oncol. 2022;40(16_suppl):3512–3512.
- Lutsyk M, Awawda M, Gourevich K, et al. Tumor volume as predictor of pathologic complete response following neoadjuvant chemoradiation in locally advanced rectal cancer. Am J Clin Oncol. 2021;44(9):482–486.
- Martens MH, van Heeswijk MM, van den Broek JJ, et al. Prospective, multicenter validation study of magnetic resonance volumetry for response assessment after preoperative chemoradiation in rectal cancer: can the results in the literature be reproduced? Int J Radiat Oncol Biol Phys. 2015;93(5):1005–1014.
- Dubben HH, Thames HD, Beck-Bornholdt HP. Tumor volume: a basic and specific response predictor in radiotherapy. Radiother Oncol. 1998;47(2):167–174.
- Johnsson A, Leon O, Gunnlaugsson A, et al. Determinants for local tumour control probability after radiotherapy of anal cancer. Radiother Oncol. 2018;128(2):380–386.
- Beets-Tan RGH, Lambregts DMJ, Maas M, et al. Magnetic resonance imaging for clinical management of rectal cancer: updated recommendations from the 2016 european society of gastrointestinal and abdominal radiology (ESGAR) consensus meeting. Eur Radiol. 2018;28(4):1465–1475.
- Glynne-Jones R. Watch and wait in rectal cancer: is it time to subclassify cT3? Lancet Gastroenterol Hepatol. 2018;3(12):814–815.
- Habr-Gama A, São Julião GP, Vailati BB, et al. Organ preservation in cT2N0 rectal cancer after neoadjuvant chemoradiation therapy: the impact of radiation therapy dose-escalation and consolidation chemotherapy. Ann Surg. 2019;269(1):102–107.
- São Julião GP, Habr-Gama A, Vailati BB, et al. Is neoadjuvant chemoradiation with dose-escalation and consolidation chemotherapy sufficient to increase surgery-free and distant metastases-free survival in baseline cT3 rectal cancer? Eur J Surg Oncol. 2018;44(1):93–99.
- Chadi SA, Malcomson L, Ensor J, et al. Factors affecting local regrowth after watch and wait for patients with a clinical complete response following chemoradiotherapy in rectal cancer (InterCoRe consortium): an individual participant data meta-analysis. Lancet Gastroenterol Hepatol. 2018;3(12):825–836.