Abstract
Background
Survival in patients with metastatic colorectal cancer (mCRC) has markedly improved in patients included in clinical trials. In population-based materials, improvements were seen until about a decade ago, but it is unclear if survival has continued to improve. It is also unclear if regional or gender differences exist.
Material and methods
All patients with mCRC (N = 19,566) in Sweden between 2007 and 2016 were identified from the national quality register, SCRCR, with almost complete coverage. Overall survival (OS) from diagnosis of metastatic disease was calculated in two calendar periods, 2007–2011 and 2012–2016. Differences between groups were compared using Cox regression.
Results
Median age was 72 years, 55% were males, synchronous presentation was seen in 13,630 patients and metachronous in 5936. In synchronous disease, the primary tumour was removed more often during the first than the second period (51% vs 41%, p < 0.001). Median OS (mOS) was 14.0 months. It was longer in those with metachronous than synchronous disease (17.6 vs 13.1 months, p < 0.001) and in males (15.0 vs 12.8 months, p < 0.001), and markedly influenced by age and primary location. It was longer in patients diagnosed during the second period than during the first (14.9 vs 13.1 months, HR 0.89 (95% CI 0.86–0.92), p < 0.001). This difference was seen in all subgroups according to sex, age, presentation, and sidedness. mOS was about one month shorter in 1/6 healthcare regions, most pronounced during the first period. Differences in median of up to 5 months were seen between the region with the shortest and longest mOS.
Conclusions
Overall survival in Swedish patients with mCRC has improved during the past decade but is still substantially worse than reported from clinical trials/hospital-based series, reflecting the selection of patients to trials. Regional differences were seen, but they decreased with time. Women did not have a poorer prognosis in multivariable analyses.
Background
Globally, colorectal cancer (CRC) is the fourth most common cancer and the second most common cancer killer [Citation1]. At diagnosis, 20–25% of the patients have distant metastases and another 20–25% will recur after curative surgery [Citation2–4]. During the past decades, medical treatment for metastatic CRC (mCRC) has developed substantially [Citation5–7]. Presently 16 drugs are approved for use in mCRC [Citation7]. These have markedly prolonged survival; in recent clinical trials, median overall survival (mOS) has been about 30 months [Citation6,Citation8,Citation9]. Surgery for metastatic disease, frequently possible today thanks to tumour regression after medical treatment, has also contributed [Citation10]. International collaborative groups’ or consensus documents state, e.g., that ‘median survival of 24–30 months can be expected for patients diagnosed with mCRC, as opposed to about 6 months some decades ago’ [Citation6] or that ‘median OS is now in the 30-month range’ [Citation11].
In contrast, poorer results have been reported from population-based materials. Generally, although improvements have been documented, mOS of around 12 months has been reported [Citation12–21]. In a Dutch study, no improvement was seen between 2008 and 2016, except possibly in patients with limited tumour burden receiving upfront metastasectomy [Citation22]. Hospital and gender variability, potentially reflecting inequalities, have been reported [Citation23–26].
Patients included in trials are selected and the representativity for the population questioned [Citation15–18,Citation20,Citation22,Citation27]. They are younger, have better performance status, less comorbidities, certain metastatic sites are underrepresented, and, in recent trials, their tumours are molecularly selected. All these factors contribute to the survival differences between populations and trial/hospital series but the relative importance of them and other potentially important factors is not known.
The aim is to describe if survival has improved in the general Swedish population during recent years and if differences exist between gender and different regions in a country with a general healthcare system having the aim to provide equal health to all individuals.
Material and methods
Patients
All patients living in Sweden (population 9.1 million in 2007, 9.9 million in 2017) with a primary colorectal adenocarcinoma diagnosed after January 1, 2007, have been registered in the nationwide Swedish Colorectal Cancer Registry (SCRCR) [Citation28,Citation29]. The coverage and reporting of staging and primary surgical treatments are close to 100% [Citation30]. The accuracy of the reporting of synchronous metastases is high (underreporting <1%, overreporting 3%) [Citation31]. Completeness of the reporting of recurrences is also high (about 1–2% missed after 5 years), but 3% are inaccurate [Citation31]. Curatively operated patients are followed clinically after one and three years with reporting to SCRCR whenever a recurrence occurred. Reporting of non-surgical treatments started in 2011 but has varied between hospitals, although it has improved and is presently about 80% [Citation32].
Surgery, radiotherapy, and chemotherapy have been given according to the national Swedish care programmes from 2008 and 2016. The principles adhere to the ESMO guidelines [Citation2,Citation6,Citation33].
An extraction of patients with mCRC from the SCRCR was made in March 2018. Patients with a registration of metastatic disease (M1) at initial diagnosis (liver, lung), after surgery if done (liver, lung, other specified) and patients registered as M0 at diagnosis with a recurrence (loco-regional or systemic [liver, lung, other specified]) during follow-up were eligible (N = 19,708). In addition, patients who had information about treatment for metastatic disease were included even if not registered as having M1 disease or recurrence (N = 708).
In Sweden, synchronous disease is present at diagnosis or at surgery, thus, no time limit for when metachronous disease appears [Citation34] is applied. Only patients with metachronous metastases were included in time to recurrence analyses. The time was measured from diagnosis of the primary tumour to time of diagnosis of metastatic disease or local recurrence, in all but a few patients with missing dates, where date of multidisciplinary team assessment or treatment initiation was used instead.
According to the TNM classifications during the period, rectal cancer patients with positive lateral or inguinal nodes were classified as M1. Since they can be treated with loco-regional therapy (surgery ± chemoradiotherapy) and have a better prognosis than those with systemic disease, analyses without them were also performed.
Patients with a diagnosis of metastasis or a locally advanced cancer not removed after attempts to resection were referred to the synchronous group (M1) and patients with M0 at diagnosis with registered recurrence after resection surgery constitute the metachronous group (Supplementary Figure 1).
Statistics
Categorical data were presented as proportions with percentages and compared using Chi-square. Survival times were calculated from the date of diagnosis, being the date of initial diagnosis if synchronous or the date when the first loco-regional or systemic failure was detected, to the date of death or end of follow-up in April 2018. In 822 patients with non-synchronous disease in the original data file, the date of recurrence was not registered. In these patients, the starting date of chemotherapy, radiotherapy or surgery for metastatic disease or the date of a multidisciplinary team conference stating a palliative approach was used. Crude OS stratified by age groups and diagnostic calendar period of the primary tumour (2007–2011 and 2012–2016) was calculated using the Kaplan–Meier method. In addition, point estimates of mOS and 5-year OS with 95% confidence intervals (CIs) were calculated.
Cox proportional hazards models were fitted to compare mortality risk rates between the two periods and different characteristics. The results are presented as hazard ratios (HRs) with 95% CIs. Proportional hazard assumption testing was done by visually assessing Schoenfeld residuals. Visually no clear violations of the proportional hazard assumption could be observed. We chose to interpret the results as a weighted average of the time-varying hazard ratios [Citation35]. P-values <0.05 were considered statistically significant and all tests were two-sided. The analyses were performed using SPSS versions 25 and 27.
Ethics
The study is conducted in accordance with the Declaration of Helsinki and approved by the ethical review board in Uppsala. Every patient has the right not to be registered in a quality registry, although this rarely happens.
Results
Characteristics of the cohort
Between January 2007 and December 2016, 19,708 patients were registered with mCRC in the SCRCR, 13,071 with synchronous disease (M1), 5930 with recurrence (initially M0) and 708 patients (all M0) with information on a ‘generalised form’ about generalised disease (actively treated or not). After scrutinisation of available information for these patients, 19,566 patients remained of which 13,630 belonged to a synchronous group and 5936 to a metachronous group (Supplementary Figure 1).
In the synchronous group, 86 rectal cancer patients with lateral nodes only, 20 patients with inguinal nodes only (M1 according to TNM 5-7) and 269 patients with a non-resectable primary tumour but without registered distant metastases were identified. In the metachronous group, 721 patients had a registered local recurrence only, 63 after an endoscopic procedure. Fifty of these had distant metastases, leaving 598 patients with a local recurrence only after a major resection. The remaining 5338 patients in the metachronous group had distant metastases. Eighty-three patients had no registered date of recurrence diagnosis and were excluded from the survival analyses (Supplementary Figure 1).
In the 19,566 patients, 10,711 (55%) were men and median age was 72 years. The primary tumour site was colon in 12,856 (66%) patients (of which 230 started in appendix, 6706 [34%] were right-sided, and 5920 [30%] were left-sided), and rectum in 6597 (34%). In 113 patients, the localisation was unknown, or multiple sites were present. Overall, 13,051 (67%) had liver metastases and 7593 (39%) lung metastases, proportions similar to an earlier Swedish study [Citation36]. Liver metastases were particularly common in patients with synchronous disease (74%) (Supplementary Table 1). They were also more common during the first calendar period (71% vs 64%, p < 0.001, ).
Table 1. Background demographics for time periods (2007–2011 vs 2012–2016) and further divided by presentation of metastases.
Resection of the primary tumour
In patients with synchronous disease, the primary tumour was removed in 6241 (46%) patients. Thus, 7389 patients had their primary tumour left in situ, however, attempts to remove the tumour (open-and-close) were done in 1439 (11%) patients. Resections were performed more often in colon than in rectal cancer (52% vs. 33%, p < 0.001), and in patients ≤70 years compared with patients >70 years at diagnosis (50% vs 43%, p < 0.001).
Among all patients, primary tumour resections and attempts to resect were more common during the first than during the second period (73% vs 66%, p < 0.001, ). Of the 5175 (81% of all synchronous) patients with known metastases at diagnosis before surgery was planned/done, 2948 (57%) were operated on between 2007–2011 and 2893 (47%, p < 0.001) of the 6176 (85% of all synchronous) between 2012 and 2016. In both periods, most patients with M0 disease at initial work-up were operated (98% vs 93%, p < 0.001), and most of them (93% vs 90%, p < 0.001) resected.
Time to recurrence
In patients with metachronous metastases, 506 (9%) developed within 6 months, 4383 (75%) between 6 months and 3 years, 841 (14%) between 3 and 5 years and 123 (2%) later. Median time to recurrence was 16 months (range 0.5–107). In patients with follow-up exceeding 5 years (primary diagnosis 2007-March 2013), these proportions were 8%, 73%, 16% and 3%. Restricting the period to those with minimum follow-up over 7 years (2007-March 2011) did not change the results (the proportion diagnosed after 5 years increased from 2.6% to 3.2%). Recurrences >5 years after primary diagnosis tended to be more common in rectal cancer than in left or right-sided colon cancer (3% vs 1% vs 2%) and slightly more common in stage II than stage III (3% vs 2%). In stage I, 5% of the few recurrences came after 5 years. Of the recurrences seen after 5 years, most had stage III and 15% were stage I (Supplementary Figure 2).
Tumour characteristics
All patients but 494 (2.5%) had a morphologically verified adenocarcinoma. This proportion did not differ between the periods (2.5% vs 2.5%, p = 0.926). The SCRCR only registers adenocarcinomas but allows registration on clinical grounds only. Patients without morphological diagnosis were older (median 73 years vs 71 years), had mostly synchronous disease (93%) and were seldom resected (N = 60 [12%], open-and-close was common [N = 54, 47% of those having surgery]). One hundred and seventy-seven of these patients received oncologic treatment; since tumours according to the national guidelines must be morphologically verified prior to this therapy, a morphological diagnosis probably occurred later but was not reported.
The degree of differentiation was only recorded if the primary tumour was resected; thus, it was missing in many patients with synchronous disease. Of those registered, about 70% had high or moderate differentiation, with no difference between the periods (, Supplementary Table 1).
Overall survival
Overall
Median OS in all patients was 14.0 (95%CI 13.7–14.4) months. It was longer in patients with metachronous disease (17.6 months) than in those with synchronous disease (13.1 months). In patients with a local recurrence only, it was 13.0 months (, ). In patients with recurrence within the first 6 months, it was as short as in those with synchronous disease and it was longest in those with a recurrence between 3 and 5 years (median almost 24 months, Supplementary Figure 3). Men had longer mOS than women (15.0 vs 12.8 months, ). OS was markedly influenced by age (, Supplementary Figure 4(A–D)). Patients with metastases from a rectal primary or a left-sided colon cancer had better OS than those with a right-sided primary (mOS 17.9 months, 16.5 months, and 9.4 months, respectively (, )). It was also better in those with lateral or inguinal nodes only (Supplementary Figure 5). Patients with lung metastases only had longer mOS than those with liver metastases, one other metastatic site only or multiple sites (mOS 29.0 vs 14.4 vs 11.5 vs 12.3 months respectively (, Supplementary Figure 6(A,B)).
Figure 1. Overall survival between patients diagnosed 2007–2011 and 2012–2016 for all patients (A), synchronous presentation (B), metachronous (excluding local recurrence only) (C), and local recurrence only (D).
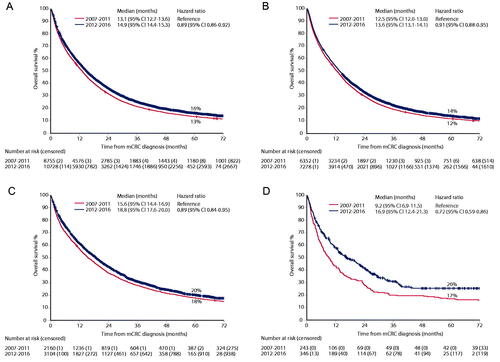
Figure 2. Overall survival by sex (A and B), primary location (C and D), and healthcare region (E and F) in the two time periods (2007–2011 [left panes] vs 2012–2016 [right panes]).
![Figure 2. Overall survival by sex (A and B), primary location (C and D), and healthcare region (E and F) in the two time periods (2007–2011 [left panes] vs 2012–2016 [right panes]).](/cms/asset/f5b3146e-6834-430f-923b-e09fca797326/ionc_a_2126327_f0002_c.jpg)
Table 2. Median overall survival and 5-year overall survival rates divided in time periods (2012–2016 vs. 2007–2011) in subgroups of age, sex, primary location, degree of differentiation, presentation of metastases, metastatic sites and healthcare region.
Patients with well/moderately differentiated tumours had better OS than patients with poor/undifferentiated tumours (, Supplementary Figure 7(A)). Patients without morphological diagnosis had shorter mOS than the remaining cases (6.6 vs 14.3 months, Supplementary Figure 7(B)).
Difference between periods
An improvement in OS was seen between the periods 2007–2011 and 2012–2016. This was seen in all patients, in patients with synchronous and metachronous disease, and in patients with one metastatic site, whether lung, liver or other, and local recurrence only, where the most marked increase was seen (, , Supplementary Figure 6(A-B)). It was also seen for both sexes (, ), all age groups (although only a minimal but statistically significant increase was seen in those over 80 years (, Supplementary Figure 4(A–D))), for all sites (, ) and when those with lateral or inguinal nodes or locally advanced disease only were excluded (Supplementary Table 2).
Difference between regions
A presentation of the organisation of the Swedish healthcare is presented in Supplementary description 1. OS differed slightly between the 6 healthcare regions (populations in 2017 899,000-2 456,000) (, ). mOS was about 1 month shorter in one healthcare region. This difference was numerically more pronounced in the first than in the second period. Looking at individual regions (populations 131,000– 2 396,000), more marked differences were seen, with a median difference of 5 months (excluding the smallest region) between the regions with the best and the worst survival (Supplementary Table 3). The differences between regions were similar during the first and the second period. The differences were seen also if synchronous and metachronous disease were analysed separately and if rectal cancers with lateral/inguinal nodes and appendix cancers were excluded (data not shown).
Multivariable analyses
Age, primary tumour location, presentation of metastases, metastatic sites, some healthcare regions and time periods were all associated with OS, whereas sex was not (). Degree of differentiation was also prognostic, but since the many missing cases were virtually only seen in synchronous disease when the primary tumour was not removed (, Supplementary Table 1), the degree of differentiation was only included in a separate analysis (Supplementary Table 4).
Table 3. Univariable and multivariable analyses for effects on overall survival, global p-values for the variables are shown after the reference category (HR = 1).
Discussion
A median survival of about 14 months in the Swedish population during a recent 10-year period markedly contrasts with the generally held view that half of the patients with mCRC today can expect to live above 2 years. It is known that patients in trials or at dedicated hospitals are selected, and not representative of the population [Citation15,Citation18,Citation22,Citation37]. This knowledge is, however, forgotten in guidelines and reviews, describing treatment recommendations and outcomes in young and fit patients [Citation6–8, Citation11, Citation38].
Overall, a slight improvement in OS was seen between the first and the second period. Since this was seen in all subgroups according to age, gender, presentation, primary tumour location, metastatic site and was most pronounced in the healthcare region having the worst survival during the first period, we believe it is true. We could not detect any methodological aspects that could explain it.
Literature is consistent in that population-based survival improvements from 5 to 8 months during the 1980–1990s to 10–12 months during the first decade of this century have been recorded, i.e., up to about 10 years ago [Citation12–14,Citation17–21,Citation39,Citation40]. In a French study, improvements were seen up to 1997–2004 but with no further improvement between 2005 and 2009 [18]. In an analysis of SEER data including 10,325 resected patients with synchronous disease, OS was better between 2013 and 2015 than between 2010 and 2012 (HR 0.94 [95%CI 0.89–1.00]) [Citation41]. In another analysis of SEER data an improvement in mOS from 12 months in 1986–1996 to 21 months (95% CI 21–22) in 2007–2015 was seen, p < 0.001 [Citation42]. In a Dutch study including 21,407 patients between 2008 and 2016, no improvement in mOS was seen, being approximately 12 months throughout the period [Citation22]. An improvement from 25 to 29 months was only seen in the group of patients having the longest survival. Our results revealing improvements in all subgroups including the oldest patients, thus, contrast to the Dutch study.
The studies reporting improvements until about 10 years ago have claimed that the development in medical oncology with several new effective drugs is chiefly responsible. Medical treatment of mCRC developed markedly some 15–30 years ago but, besides the recent addition of BRAF- and immune checkpoint inhibitors [Citation43,Citation44] of no relevance for the present study, no new effective drugs have been added to the armamentarium since about 2007 when EGFR-inhibiting antibodies were registered. One additional cytotoxic drug, trifluridine-tipiracil and a tyrosine kinase inhibitor, regorafenib, have been approved, but they are only used in later lines and have minimal influence on OS, a few months in the trials. Better OS in the most recent trials compared to those reported 10–15 years ago [Citation6,Citation8,Citation38] can probably be explained by either more optimal use of the drugs or better patient selection with exclusion of patient groups/tumour types with the worst survival. RAS- and particularly BRAF-mutant tumours carry a worse prognosis than wild-type tumours, and the trials reporting the longest survival have only included wild-type tumours since an EGFR-inhibitor was tested [Citation6].
Development in medical oncology in mCRC has favoured patients with tumours arising in left-sided cancers. Similar to other studies, OS was shorter in right-sided colon cancers. This was true during both periods, but the relative improvement did not differ according to sidedness (HR about 0.90 for both). Since right-sided cancers much more often than left-sided are BRAF-V600E-mutated or have a deficient mismatch repair system [Citation45], the two major groups presently with the worst survival, the difference according to sidedness is easy to understand. It could possibly diminish due to the recent approval of more effective therapies for these uncommon subgroups [Citation43, Citation44].
The distinction between synchronous and metachronous disease is controversial and handled differently between countries [Citation34,Citation46]. Our results show that from a prognostic view, recurrences within the first 6 months after primary surgery have the same prognosis as ‘truly’ synchronous disease.
Treatment after diagnosis of metastatic disease was not registered in the SCRCR until 2011 and was initially sparsely registered. It has improved but is still incomplete [Citation32]. Although information about initial treatment is available in many patients (8% of those diagnosed 2007–2011, 46% 2012–2016), it is not possible to make proper analyses since the information is not representative of the population. Thus, the probably most important reason for the improvement seen during the second period and for the differences between healthcare regions/regions, i.e., the use of different drugs, is not possible to evaluate. In a Dutch study exploring patients treated between 2008 and 2015, marked differences in the use of bevacizumab were seen according to type of hospital [Citation24]. The study patients treated with bevacizumab had better OS than patients not treated after adjustment for known co-variables, similar to other reports [Citation47–49].
Thanks to the introduction of combination chemotherapy around 2000 and the addition of biologic agents some years later, surgery of metastases has been increasingly used contributing to further survival improvements [Citation50–56]. An important quality aspect of relevance for survival, at least long-term survival is, thus, the number of patients evaluated for metastatic surgery and then operated. Studies in Sweden have noted that inequalities between hospitals exist in the proportions referred to liver metastatic surgery and operated upon, but no knowledge is present if this has changed during the investigated time [Citation52,Citation54]. It is possible that this may have contributed to the improvement with time in long-term survival reported here (5-year OS rates 14% vs 16%) [Citation50–53,Citation55].
One aspect of potential importance and possible to explore here is the decrease in resection rates of the primary tumour in patients with synchronous metastases. Whether resection of the primary tumour prolongs survival is debated [Citation57]. Multiple retrospective analyses have not reached firm conclusions and conclusive randomised studies are not present. One Swedish attempt failed [Citation58]. In the Dutch CAIRO-4 trial (NCT01606098), initial results show that death within 60 days is higher in patients randomised to primary surgery [Citation59]. If the lower resection rate during the second period, permitting earlier initiation of systemic therapy, is important for the improvement seen here is not known but possible in the light of the Dutch short-term data.
Strengths and limitations
The SCRCR has in a national and international perspective high validity and can provide internationally competitive information about the quality of the care in the entire nation. Its coverage is almost 100% and many key variables including registration of synchronous metastases is well covered [Citation30]. Of interest here is that the registration of clinical and pathological M-stage is accurate in 96%. In a detailed evaluation of accuracy and completeness of registration of synchronous metastases and recurrences, resulting in metachronous metastases, in one (synchronous metastases) and two (recurrences) regions, errors were found in between 1 and 3% [Citation31]. The evaluation of the registered information in the datafile obtained for this study resulted in that several potential errors were found and corrected prior to analyses. Thus, it is our belief that the quality of the data is at a very high level. All population registries, including this one, contain errors but the extent of these is in all probability small, not challenging either the overall results, the improvements seen between the two periods and the lack of major differences during the second period between healthcare regions. The lack of detailed information about oncological treatments is disadvantageous, making it difficult to interpret the reasons behind the improved OS with time, both overall and between subgroups.
Conclusions
This study substantiates the major differences in OS between the mCRC population and what is seen in clinical trials and written in guidelines. These are to a great extent reflecting what can be achieved in the (minority) of patients fit enough to take advantage of the most recent development in therapy. Despite this, an improvement was seen from the first to the second period. This is not caused by availability of new treatments, rather, existing treatments may be used more appropriately. The national care programmes and the yearly reports from the quality registration probably contribute to this improvement. At the same time, despite the distributed responsibility in the care of cancer patients in Sweden, these have most probably also contributed to that survival does not differ considerably between different Swedish regions. The yearly reports [Citation32] tell that treatments differ, e.g., in the choice of first-line regimen, but this does apparently not have any major impact on survival. The reports repeatedly discuss whether differences potentially reflecting imbalances in the care exist.
Supplemental Material
Download PDF (1.2 MB)Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Schmoll HJ, Van Cutsem E, Stein A, et al. ESMO consensus guidelines for management of patients with Colon and rectal cancer. a personalized approach to clinical decision making. Ann Oncol. 2012;23(10):2479–2516.
- Osterman E, Ekstrom J, Sjoblom T, et al. Accurate population-based model for individual prediction of Colon cancer recurrence. Acta Oncol. 2021;60(10):1241–1249.
- Osterman E, Hammarstrom K, Imam I, et al. Recurrence risk After radical colorectal cancer surgery–less Than Before, But how high Is It? Cancers. 2020;12(11):3308.
- Glimelius B, Cavalli Björkman N. Metastatic colorectal cancer: Current treatment and future options for improved survival. Medical approach–present status. Scand J Gastroenterol. 2012;47(3):296–314.
- Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386–1422.
- Biller LH, Schrag D. Diagnosis and treatment of metastatic colorectal cancer: a review. JAMA. 2021;325(7):669–685.
- Venook AP, Niedzwiecki D, Lenz HJ, et al. Effect of First-Line chemotherapy combined With cetuximab or bevacizumab on overall survival in patients With KRAS Wild-Type advanced or metastatic colorectal cancer: a randomized clinical trial. JAMA. 2017;317(23):2392–2401.
- Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab or bevacizumab for advanced colorectal cancer: final survival and per-protocol analysis of FIRE-3, a randomised clinical trial. Br J Cancer. 2021;124(3):587–594.
- Adam R, Kitano Y. Multidisciplinary approach of liver metastases from colorectal cancer. Ann Gastroenterol Surg. 2019;3(1):50–56.
- Atreya CE, Yaeger R, Chu E. Systemic therapy for metastatic colorectal cancer: from current standards to future molecular targeted approaches. Am Soc Clin Oncol Educ Book. 2017;37:246–256.
- Lambert PC, Dickman PW, Osterlund P, et al. Temporal trends in the proportion cured for cancer of the Colon and rectum: a population-based study using data from the finnish cancer registry. Int J Cancer. 2007;121(9):2052–2059.
- Meulenbeld HJ, van Steenbergen LN, Janssen-Heijnen ML, et al. Significant improvement in survival of patients presenting with metastatic Colon cancer in the South of The Netherlands from 1990 to 2004. Ann Oncol. 2008;19(9):1600–1604.
- Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27(22):3677–3683.
- Sorbye H, Pfeiffer P, Cavalli-Bjorkman N, et al. Clinical trial enrollment, patient characteristics, and survival differences in prospectively registered metastatic colorectal cancer patients. Cancer. 2009;115(20):4679–4687.
- Lemmens VE, de Haan N, Rutten HJ, et al. Improvements in population-based survival of patients presenting with metastatic rectal cancer in the South of The Netherlands, 1992-2008. Clin Exp Metastasis. 2011;28(3):283–290.
- Sorbye H, Cvancarova M, Qvortrup C, et al. Age-dependent improvement in median and long-term survival in unselected population-based nordic registries of patients with synchronous metastatic colorectal cancer. Ann Oncol. 2013;24(9):2354–2360.
- Mitry E, Rollot F, Jooste V, et al. Improvement in survival of metastatic colorectal cancer: are the benefits of clinical trials reproduced in population-based studies? Eur J Cancer. 2013;49(13):2919–2925.
- Castleberry AW, Guller U, Tarantino I, et al. Discrete improvement in racial disparity in survival among patients with stage IV colorectal cancer: a 21-year population-based analysis. J Gastrointest Surg. 2014;18(6):1194–1204.
- Ghiringhelli F, Hennequin A, Drouillard A, et al. Epidemiology and prognosis of synchronous and metachronous Colon cancer metastases: a french population-based study. Dig Liver Dis. 2014;46(9):854–858.
- van der Geest LG, Lam-Boer J, Koopman M, et al. Nationwide trends in incidence, treatment and survival of colorectal cancer patients with synchronous metastases. Clin Exp Metastasis. 2015;32(5):457–465.
- Hamers PAH, Elferink MAG, Stellato RK, et al. Informing metastatic colorectal cancer patients by quantifying multiple scenarios for survival time based on real-life data. Int J Cancer. 2021;148(2):296–306.
- Krell RW, Regenbogen SE, Wong SL. Variation in hospital treatment patterns for metastatic colorectal cancer. Cancer. 2015;121(11):1755–1761.
- Keikes L, Koopman M, Stuiver MM, et al. Practice variation on hospital level in the systemic treatment of metastatic colorectal cancer in The Netherlands: a population-based study. Acta Oncol. 2020;59(4):395–403.
- Lao C, Kuper-Hommel M, Laking G, et al. Evidence of inequitable use of chemotherapy in New Zealand colorectal cancer patients. N Z Med J. 2020;133(1520):15–26.
- White A, Ironmonger L, Steele RJC, et al. A review of sex-related differences in colorectal cancer incidence, screening uptake, routes to diagnosis, cancer stage and survival in the UK. BMC Cancer. 2018;18(1):906.
- Ludmir EB, Mainwaring W, Lin TA, et al. Factors associated With age disparities Among cancer clinical trial participants. JAMA Oncol. 2019;5(12):1769–1773.
- Kodeda K, Nathanaelsson L, Jung B, et al. Population-based data from the swedish Colon cancer registry. Br J Surg. 2013;100(8):1100–1107.
- RCC. Nationella kvalitetsregister cancer. https://wwwcancercentrumse/sydost/vara-uppdrag/kunskapsstyrning/kvalitetsregister/. 2020.
- Moberger P, Skoldberg F, Birgisson H. Evaluation of the swedish colorectal cancer registry: an overview of completeness, timeliness, comparability and validity. Acta Oncol. 2018;57(12):1611–1621.
- Osterman E, Hammarstrom K, Imam I, et al. Completeness and accuracy of the registration of recurrences in the swedish colorectal cancer registry (SCRCR) and an update of recurrence risk in Colon cancer. Acta Oncol. 2021;Mar 9:1–8. Online ahead of print. PMID: 33689551.
- RCC. Regionala rapporter. https://wwwcancercentrumse/norr/cancerdiagnoser/tjocktarm-andtarm-och-anal/tjock–och-andtarm/kvalitetsregister/. 2020;Accessed Dec 8, 2020.
- Van Cutsem E, Nordlinger B, Cervantes A, ESMO Guidelines Working Group, et al. Advanced colorectal cancer: ESMO clinical practice guidelines for treatment. Ann Oncol. 2010;21 Suppl 5:v93–7.
- Goey KK, t Lam-Boer J, de Wilt JH, et al. Significant increase of synchronous disease in first-line metastatic colorectal cancer trials: Results of a systematic review. Eur J Cancer. 2016;69:166–177.
- Stensrud MJ, Hernán MA. Why test for proportional hazards? Jama. 2020;323(14):1401–1402.
- Riihimaki M, Hemminki A, Sundquist J, et al. Patterns of metastasis in Colon and rectal cancer. Sci Rep. 2016;6:29765.
- Unger JM, Barlow WE, Martin DP, et al. Comparison of survival outcomes among cancer patients treated in and out of clinical trials. J Natl Cancer Inst. 2014;106(3):dju002.
- Heinemann V, Stintzing S. FOLFIRI with cetuximab or bevacizumab: FIRE-3-Authors’ reply. Lancet Oncol. 2014;15(13):e583-4–e584.
- Platell C, Ng S, O'Bichere A, et al. Changing management and survival in patients with stage IV colorectal cancer. Dis Colon Rectum. 2011;54(2):214–219.
- Golan T, Urban D, Berger R, et al. Changing prognosis of metastatic colorectal adenocarcinoma: Differential improvement by age and tumor location. Cancer. 2013;119(16):3084–3091.
- Siebenhuner AR, Guller U, Warschkow R. Population-based SEER analysis of survival in colorectal cancer patients with or without resection of lung and liver metastases. BMC Cancer. 2020;20(1):246.
- Shen C, Tannenbaum D, Horn R, et al. Overall survival in phase 3 clinical trials and the surveillance, epidemiology, and end results database in patients With metastatic colorectal cancer, 1986-2016: a systematic review. JAMA Netw Open. 2022;5(5):e2213588.
- Sahin IH, Klostergaard J. BRAF mutations as actionable targets: a paradigm shift in the management of colorectal cancer and novel avenues. J Clin Oncol Oncol Pract. 2021;17(12):723–730.
- Damilakis E, Mavroudis D, Sfakianaki M, et al. Immunotherapy in metastatic colorectal cancer: Could the latest developments hold the key to improving patient survival? Cancers (Basel). 2020;12(4):889.
- Nunes L, Aasebo K, Mathot L, et al. Molecular characterization of a large unselected cohort of metastatic colorectal cancers in relation to primary tumor location, rare metastatic sites and prognosis. Acta Oncol. 2020;59(4):417–426.
- Kumar R, Price TJ, Beeke C, et al. Colorectal cancer survival: An analysis of patients with metastatic disease synchronous and metachronous with the primary tumor. Clin Colorectal Cancer. 2014;13(2):87–93.
- Hammerman A, Greenberg-Dotan S, Battat E, et al. The 'real-life’ impact of adding bevacizumab to first-line therapy in metastatic colorectal cancer patients: a large israeli retrospective cohort study. Acta Oncol. 2015;54(2):164–170.
- Stein A, Petersen V, Schulze M, et al. Bevacizumab plus chemotherapy as first-line treatment for patients with metastatic colorectal cancer: results from a large german community-based observational cohort study. Acta Oncol. 2015;54(2):171–178.
- Tomita Y, Karapetis CS, Ullah S, et al. Survival improvements associated with access to biological agents: Results from the South Australian (SA) metastatic colorectal cancer (mCRC) registry. Acta Oncol. 2016;55(4):480–485.
- Young AL, Adair R, Culverwell A, et al. Variation in referral practice for patients with colorectal cancer liver metastases. Br J Surg. 2013;100(12):1627–1632.
- Hackl C, Neumann P, Gerken M, et al. Treatment of colorectal liver metastases in Germany: a ten-year population-based analysis of 5772 cases of primary colorectal adenocarcinoma. BMC Cancer. 2014;14:810.
- Noren A, Eriksson HG, Olsson LI. Selection for surgery and survival of synchronous colorectal liver metastases; a nationwide study. Eur J Cancer. 2016;53:105–114.
- Angelsen JH, Horn A, Sorbye H, et al. Population-based study on resection rates and survival in patients with colorectal liver metastasis in Norway. Br J Surg. 2017;104(5):580–589.
- Noren A, Sandstrom P, Gunnarsdottir K, et al. Identification of inequalities in the selection of liver surgery for colorectal liver metastases in Sweden. Scand J Surg. 2018;107(4):294–301.
- Engstrand J, Nilsson H, Stromberg C, et al. Colorectal cancer liver metastases - a population-based study on incidence, management and survival. BMC Cancer. 2018;18(1):78.
- Adam L, San Lucas FA, Fowler R, et al. DNA sequencing of small bowel adenocarcinomas identifies targetable recurrent mutations in the ERBB2 signaling pathway. Clin Cancer Res. 2019;25(2):641–651.
- Nitsche U, Stöß C, Stecher L, et al. Meta-analysis of outcomes following resection of the primary tumour in patients presenting with metastatic colorectal cancer. Br J Surg. 2018;105(7):784–796.
- Arbman G, Pahlman L, Glimelius B. The rise and fall of a longed for clinical trial in patients with generalized colorectal cancer. Acta Oncol. 2013;52(8):1779–1782.
- van der Kruijssen DEW, Elias SG, Vink GR, CAIRO4 Working Group, et al. Sixty-Day mortality of patients With metastatic colorectal cancer randomized to systemic treatment vs primary tumor resection followed by systemic treatment: the CAIRO4 phase 3 randomized clinical trial. JAMA Surg. 2021;156(12):1093–1101.
