Introduction
Immune checkpoint inhibitors (ICIs) have been increasingly used as a monotherapy or in combination with chemotherapy in the frontline setting for various cancers. Despite significant clinical benefits, ICIs are associated with a unique spectrum of adverse effects termed immune-related adverse events (irAEs). Caused by nonspecific activation of the immune system, irAEs can affect multiple organs, including skin, endocrine, gastrointestinal tract, lungs, and liver [Citation1].
However, little data is available regarding the safety of ICIs in patients undergoing peritoneal dialysis or hemodialysis, or end-stage renal disease (ESRD), as most clinical trials have excluded this population. Indeed, the current package inserts for pembrolizumab and nivolumab do not provide information regarding safety data or dose adjustments in patients with an estimated glomerular filtration rate (eGFR) <15 mL/min/1.73 m2, a range corresponding to chronic kidney disease (CKD) stage 5 or ESRD [Citation2,Citation3]. Nevertheless, patients with ESRD are at a higher risk of developing cancer and are associated with much worse survival outcomes [Citation4,Citation5]. Therefore, real world data regarding the safety and efficacy of ICIs in this population are urgently needed.
However, no large-scale data are available to examine various irAEs across different cancer types. In this study, through an aggregated electronic health record (EHR) database, we investigated five of the most common irAEs associated with PD-1 inhibitors pembrolizumab and nivolumab—skin toxicity, thyroid toxicity, colitis, pneumonitis, and hepatitis—in matched patients with or without ESRD who had melanoma, renal cell carcinoma (RCC), lung, head/neck, or bladder cancer, where PD-1 inhibitors are widely used in the frontline setting for metastatic diseases. We also compared overall survival (OS) for matched patients with or without ESRD who received pembrolizumab or nivolumab.
Methods
Data were collected from an aggregated EHR database TriNetX Research Network (TriNetX LLC., Cambridge, Massachusetts, USA), which provided access to the diagnoses, procedures, medications, laboratory values, and genomic information of more than 80 million patients from 58 healthcare organizations.
Adult patients with a diagnosis of lung, renal cell, bladder, head/neck cancer, or melanoma, with pembrolizumab or nivolumab initiated between January 2018 and March 2021, were included. Patients were followed until the date of analysis on 10 March 2022. irAEs were recorded in the first year following PD-1 inhibitors initiation as they are rare after the first year [Citation6]. A 1:1 propensity score matching with baseline factors including age, sex, race, use of pembrolizumab or nivolumab, and concurrent use of ipilimumab, was applied to all cancer types.
International Classification of Disease 10 Clinical Modification (ICD-10-CM) codes C64, C43/C44, C34, C67, and C00-14 were used to identify RCC, melanoma, lung cancer, bladder cancer, and head/neck cancer in the database, respectively. RxNorm code 1547545 and Healthcare Common Procedure Coding System (HCPCS) code J9271 were used to identify pembrolizumab use. RxNorm code 1597876 and HCPCS code J9299 were used to identify nivolumab use. ICD-10-CM codes N18.5, N19.6, and Z99.2 were used to identify patients with ESRD. A set of ICD-10-CM codes that have been previously validated to identify irAEs were used with minor modifications [Citation7]. Specifically, R21 and L80 were used to identify skin toxicities; E03.2, E03.8, E03.9, and E05 were used to identify thyroid dysfunction; K52.3, K52.8, and K52.9 were used to identify colitis; J70.2, J70.3, J70.4, J70.8, J84.11, and J84.9 were used to identify pneumonitis; K71, K75.4, and K75.9 were used to identify hepatitis.
Statistical analysis
A greedy algorithm with the nearest neighbor method was used for the propensity score matching. Cumulative events and OS were calculated using the Kaplan-Meier method; Log-rank tests were performed to test for significance at a two-sided alpha level of 0.05, and Cox models were used to construct 95% confidence intervals (CIs) for the hazard ratio. For irAEs with an incidence <10, the exact number of events was inferred from the Kaplan-Meier curves. All tests were two-sided and a p-value ≤ 0.05 was considered statistically significant. Statistical calculations and graphs were created using R statistical software version 4.1.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics of patients
From January 2018 to March 2021, we identified a total of 788 recipients of pembrolizumab or nivolumab who had ESRD at the time of PD-1 inhibitors initiation (ESRD group), and a total of 37,987 recipients who never had ESRD (non-ESRD group). Their baseline characteristics were shown in Supplemental Table S1. After matching, there were 777 patients in each group, with 304 each with RCC, 190 each with lung cancer, 130 each with melanoma, 124 each with bladder cancer, and 29 each with head/neck cancer. The two groups were well balanced regarding their demographics and prior treatments ().
Table 1. Baseline characteristics of patients.
Incidence of PD-1 inhibitors related toxicities
With a median of 6 cycles of PD-1 inhibitors, 292 (37.6%) patients in the ESRD group and 276 (35.5%) in the non-ESRD group developed irAEs. Among them, 101 (13.0%) patients in the ESRD group and 86 (11.1%) in the non-ESRD group developed skin toxicities; 101 (13.0%) with ESRD and 112 (14.4%) with non-ESRD developed thyroid toxicities; 53 (6.8%) with ESRD and 49 (6.3%) with non-ESRD developed colitis; 14 (1.8%) with ESRD and 11 (1.4%) with non-ESRD developed pneumonitis; and 23 (3.0%) with ESRD and 18 (2.3%) with non-ESRD developed hepatitis (Supplemental Table S2). There was no significant difference between the two groups regarding all included toxicities (HR 1.06, 95% CI 0.89 to 1.24, p = 0.52), skin toxicities (HR 1.20, 95% CI 0.90 to 1.60, p = 0.21), thyroid toxicities (HR 0.88, 95% CI 0.67 to 1.15, p = 0.36), colitis (HR 1.09, 95% CI 0.74 to 1.61, p = 0.66), pneumonitis (HR 1.27, 95% CI 0.57 to 2.81, p = 0.55), or hepatitis (HR 1.23, 95% CI 0.66 to 2.30, p = 0.51; ). There was also no significant difference regarding the treatments patients received for irAE between the two groups (p = 0.49, Supplemental Table S2).
Figure 1. Cumulative incidence of irAEs in patients with and without ESRD. For patients with various types of cancer, there was no significant difference in cumulative incidence regarding all irAE (A), skin toxicity (B), thyroid toxicity (C), colitis (D), pneumonitis (E), or hepatitis (F).
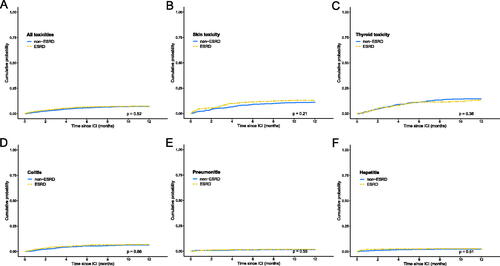
Looking into specific cancer types, the incidence of all included toxicities was not significantly different between the two groups for bladder cancer, head/neck, lung cancer, melanoma, or RCC (Supplemental Figure S1).
OS for PD-1 inhibitors recipients
Next, we compared OS for PD-1 inhibitor recipients with or without ESRD. Patients with ESRD had significantly worse OS than patients without ESRD for all included cancer types (HR 1.65, 95% CI 1.45 to 1.89, p < 0.001), bladder cancer (HR 1.78, 95% CI 1.27 to 2.47, p < 0.001), lung cancer (HR 1.59, 95% CI 1.24 to 2.03, p = 0.002), melanoma (HR 1.76, 95% CI 1.26 to 2.45, p < 0.001), or RCC (HR 1.67, 95% CI 1.33 to 2.09, p < 0.001); there was no significant difference regarding OS for head/neck cancer (HR 1.30, 95% CI 0.67 to 2.51; Supplemental Figure S2).
Sensitivity analysis
For the sensitivity analysis, all irAEs occurring in the first two weeks were excluded, because irAEs are rare in the first two weeks as shown in a meta-analysis [Citation6]. Similar to previous comparisons, there was no significant difference of various irAEs (Supplemental Figure S3).
Discussion
In this study using a large real-world database, we showed that patients with ESRD receiving PD-1 inhibitors pembrolizumab or nivolumab were not associated with an increased risk of irAE, specifically skin toxicity, thyroid toxicity, colitis, pneumonitis, or hepatitis, compared with matched patients without ESRD.
ICIs are comprised of human or humanized IgG1 antibodies, which have minimal pharmacokinetic changes in the setting of renal impairment [Citation8]. Accordingly, they also have long half-lives with receptor-mediated clearance through endocytosis or pinocytosis by the endothelial reticulum system [Citation8]. However, pharmacokinetics is poorly associated with the efficacy or safety of ICIs [Citation9]. Patients with ESRD represent a distinct population characterized by chronic systemic inflammation and impaired innate and adaptive immune systems; they harbor elevated pro-inflammatory markers and an exhausted T-cell phenotype [Citation10,Citation11]. Therefore, it is unknown whether they have a different irAE profile than patients without ESRD. Although recent case reports and small case series have suggested good safety profiles in patients with ESRD [Citation12–17], no large-scale studies have investigated the safety and efficacy of ICIs in matched cohorts of patients with and without ESRD.
The toxicity event rates observed in our study are comparable to past studies. In a meta-analysis of clinical trials using PD-1 inhibitors across different types of cancer, the incidence of any grade irAE occurred in 20% of patients receiving pembrolizumab and 48% receiving nivolumab [Citation18], on a par with our overall incidence of around 36%. Skin toxicities are the most common irAE occurring in more than one-third of patients in clinical trials [Citation19]. However, its incidence was much lower in real-world studies, likely due to insufficient charting [Citation20]. Thyroid dysfunction is the second most common irAE associated with PD-1 inhibitor therapies, occurring in 7-21% of patients [Citation21], similar to our incidence of 14%. The incidence of other less common irAEs, colitis, hepatitis, and pneumonitis, ranged from 2% to 13%, 1% to 2%, and 1% to 5% in previous studies, respectively [Citation22], comparable with incidences in this study. These comparisons suggested that although the ICD-10-CM codes did not include specific entities for different irAEs, our validated sets of codes were able to capture irAEs comprehensively.
Moreover, our study showed that although the incidences of irAEs were comparable between ESRD and non-ESRD groups, patients with ESRD receiving PD-1 inhibitors had significantly worse OS than those without ESRD. Due to limitations of the database, we were unable to assess if this was due to lower response rates, shorter response duration, or other factors influencing the efficacy of PD-1 inhibitors in this population. However, past case series have suggested that the efficacy in this population was likely not affected [Citation23]. On the other hand, ESRD itself is associated with significant morbidity and mortality, and likewise has been shown to have worse survival than patients with common solid-organ cancers [Citation24]. In addition, cancer patients with CKD grade 4 or higher had almost double mortality rates compared to those with intact renal function [Citation4].
There are several limitations in our study. Firstly, this study was retrospective in nature; although patients are matched with common risk factors for irAEs, other factors that could affect irAE rates may not have been accounted for, or adequately matched between groups. Additionally, cancer staging data and treatment history were not available for every patient. Despite our attempts to match cancer staging by identifying previous similar treatments, some discrepancies may exist. Further, there are no specific ICD-10-CM codes that specifically represent irAE. However, we used validated sets of ICD-10-CM codes for irAEs and the incidences of irAEs were comparable with past studies. Moreover, the causes of death were unavailable in the database. Therefore, it is unknown if the worse OS in the ESRD group was related to irAE. Lastly, the paucity of information regarding the grading of irAE, as well as the clinical outcomes for irAE, is crucial to keep in mind.
Conclusion
In conclusion, patients with or without ESRD receiving PD-1 inhibitors pembrolizumab or nivolumab have similar incidences of various types of irAEs. Cancer patients receiving PD-1 inhibitors who also had ESRD were shown to have significantly worse OS compared to patients without ESRD.
Author contributions
Concept and design: JW, YK
Data collection and analysis: JW
Manuscript writing: All authors
Final approval of manuscript: All authors
Supplemental Material
Download MS Word (372 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data available on request from the authors.
References
- Champiat S, Lambotte O, Barreau E, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol. 2016;27(4):559–574.
- Keytruda (pembrolizumab) [package insert]. Kenilworth, NJ: Merck & Co., Inc; 2/2022.
- Opdivo (nivolumab) [package insert]. New York, NY: Bristol Myers Squibb; 3/2022.
- Na SY, Sung JY, Chang JH, et al. Chronic kidney disease in cancer patients: an independent predictor of cancer-specific mortality. Am J Nephrol. 2011;33(2):121–130.
- Maisonneuve P, Agodoa L, Gellert R, et al. Cancer in patients on dialysis for end-stage renal disease: an international collaborative study. Lancet 1999;354(9173):93–99.
- Tang SQ, Tang LL, Mao YP, et al. The pattern of time to onset and resolution of Immune-Related adverse events caused by immune checkpoint inhibitors in cancer: a pooled analysis of 23 clinical trials and 8,436 patients. Cancer Res Treat. 2021;53(2):339–354.
- Cathcart-Rake EJ, Sangaralingham LR, Henk HJ, et al. A population-based study of immunotherapy-related toxicities in lung cancer. Clin Lung Cancer. 2020;21(5):421–427 e2.
- Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84(5):548–558.
- Le Louedec F, Leenhardt F, Marin C, et al. Cancer immunotherapy dosing: a pharmacokinetic/pharmacodynamic perspective. Vaccines (Basel). 2020;8(4):632.
- Betjes MG. Immune cell dysfunction and inflammation in end-stage renal disease. Nat Rev Nephrol. 2013;9(5):255–265.
- Hartzell S, Bin S, Cantarelli C, et al. Kidney failure associates with T cell exhaustion and imbalanced follicular helper T cells. Front Immunol. 2020;11:583702.
- Centanni M, Moes D, Troconiz IF, et al. Clinical pharmacokinetics and pharmacodynamics of immune checkpoint inhibitors. Clin Pharmacokinet. 2019;58(7):835–857.
- Kuo MC, Su PJ, Huang CC, et al. Safety and efficacy of immune checkpoint inhibitors for patients with metastatic urothelial carcinoma and End-Stage renal disease: Experiences from Real-World practice. Front Oncol. 2020;10:584834.
- Jain J, Stein J, Garje R. Evaluation of checkpoint inhibitors in cancer patients with end-stage renal disease on hemodialysis: Case series and review of the literature. J Immunother. 2020;43(8):244–249.
- Vitale MG, Baldessari C, Milella M, et al. Immunotherapy in Dialysis-Dependent cancer patients: Our experience in patients with metastatic renal cell carcinoma and a review of the literature. Clin Genitourin Cancer. 2019;17(5):e903–e908.
- Tachibana H, Kondo T, Ishihara H, et al. Safety and efficacy of nivolumab in patients with metastatic renal cell carcinoma and end-stage renal disease at 2 centers. Clin Genitourin Cancer. 2019;17(4):e772–e778.
- Cheun H, Kim M, Lee H, et al. Safety and efficacy of immune checkpoint inhibitors for end-stage renal disease patients undergoing dialysis: a retrospective case series and literature review. Invest New Drugs. 2019;37(3):579–583.
- Wang PF, Chen Y, Song SY, et al. Immune-related adverse events associated with anti-PD-1/PD-L1 treatment for malignancies: a meta-analysis. Front Pharmacol. 2017;8:730.
- Apalla Z, Papageorgiou C, Lallas A, et al. Cutaneous adverse events of immune checkpoint inhibitors: a literature review. Dermatol Pract Concept. 2021;11(1):e2021155.
- Hsiehchen D, Watters MK, Lu R, et al. Variation in the assessment of immune-related adverse event occurrence, grade, and timing in patients receiving immune checkpoint inhibitors. JAMA Netw Open. 2019;2(9):e1911519.
- Kotwal A, Kottschade L, Ryder M. PD-L1 Inhibitor-Induced thyroiditis is associated with better overall survival in cancer patients. Thyroid. 2020;30(2):177–184.
- Kennedy LB, Salama AKS. A review of cancer immunotherapy toxicity. CA Cancer J Clin. 2020;70(2):86–104.
- Kitchlu A, Jhaveri KD, Sprangers B, et al. Immune checkpoint inhibitor use in patients with end-stage kidney disease: an analysis of reported cases and literature review. Clin Kidney J. 2021;14(9):2012–2022.
- Naylor KL, Kim SJ, McArthur E, et al. Mortality in incident maintenance dialysis patients versus incident solid organ cancer patients: a Population-Based cohort. Am J Kidney Dis. 2019;73(6):765–776.
