Abstract
Background
Accurate primary staging is one of the most important issues for initial management of prostate cancer (PCa) patients to perform an optimal selection of patients for curative intended treatment. 68Ga-Prostate-Specific-Membrane-Antigen (PSMA) PET/CT was found superior to conventional imaging both for detection of recurrence after curative intended treatment and for primary staging. We studied the recurrence rate after radical prostatectomy in high-risk PCa patients primary staged with 68Ga-PSMA PET/CT compared with conventional imaging.
Material and methods
The study included 247 D’Amico high-risk PCa patients treated with radical prostatectomy (RP) after primary staging with 68Ga-PSMA PET/CT and a reference group of 137 high-risk patients with RP after conventional imaging (99mTc bone scintigraphy and CT). Recurrence rates were assessed by Cox regression and Kaplan-Meier analysis.
Results
The 5-year recurrence-free survival rate was 71.1% in the 68Ga-PSMA PET/CT cohort compared with 56.4% in the conventional imaging cohort. Primary staging by 68Ga-PSMA PET/CT reduced biochemical recurrence (BCR) risk by 42% (HR = 0.58 (0.41–0.83), p = .004).
Conclusion
The present data could indicate a lower recurrence rate after RP following primary staging with 68Ga-PSMA PET/CT compared to conventional imaging, likely due to improved selection of patients for surgery.
Background
Prostate cancer (PCa) is the second most diagnosed cancer and the fifth leading cause of cancer-related death in men worldwide [Citation1] as well as a textbook example of cancer heterogeneity. Accurate primary staging is one of the most important issues for initial management of patients [Citation2]. By now, prostate-specific membrane antigen (PSMA) is a well-established target for clinical imaging of PCa patients. Last year, the proPSMA trial [Citation3] concluded that 68Ga-PSMA-PET/CT is a suitable replacement for conventional imaging in primary staging, providing superior accuracy, to the combined findings of 99mTc bone scintigraphy and CT in D’Amico high-risk patients as conventional imaging before curative-intent radical prostatectomy (RP) or radiotherapy.
While recognizing the excellent accuracy of 68Ga-PSMA-PET/CT in the primary staging and recurrent setting, it is not clear whether improved primary staging by 68Ga-PSMA-PET/CT contributes to longer biochemical recurrence (BCR)-free survival in PCa patients after RP. This is of great importance as a substantial proportion of high-risk patients undergoing curative-intented RP will later experience recurrence following surgery depending on several risk factors [Citation4]. Therefore, the aim of this retrospective study was to investigate the recurrence rate after RP following primary staging with 68Ga-PSMA PET/CT compared with 99mTc bone scintigraphy and CT-TAP (thorax, abdomen, pelvis) as conventional imaging.
Materials and methods
Study population
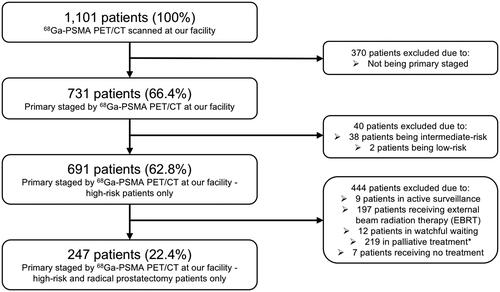
In this study, the inclusion criteria were newly diagnosed, biopsy-proven, high-risk PCa patients (according to the D’Amico classification [Citation5]) who were treated with curative-intent RP following primary staging by 68Ga-PSMA PET/CT. A total of 1,101 PCa patients were referred for 68Ga-PSMA PET/CT scans at the Department of Nuclear Medicine and PET-Centre, Aarhus University Hospital, Denmark, between April 2016 and March 2019. In this cohort, 731 patients (66.4%) were referred for primary staging, whereas the remaining 370 patients (33.6%) were referred for recurrent disease. Of the primary staged patients, 691 individuals (62.8%) were deemed high-risk. Lastly, 247 patients with localized disease (22.4%) were treated with RP following the 68Ga-PSMA PET/CT scan and thus included in the present study (). Initial findings of 68Ga-PSMA PET/CT primary staging on this cohort has previously been published [Citation6].
Clinicopathological data
Clinical and pre-operative data were gathered from medical records (Columna Clinical Information System (MidtEPJ), Systematic, Aarhus, Denmark) as well as the pathology reports from the RP specimens. The data were afterwards stored in a Research Electronic Data Capture (REDCap) database hosted by Aarhus University [Citation7]. All 247 patients underwent RP with 177 patients having a pelvic lymph node dissection within the standard template of resection according to the European Association of Urology [Citation8] based on their pre-operative risk of lymph node involvement (>7%) assessed by the Briganti nomogram [Citation9]. A total of 30 patients (12.1%) experienced BCR after positive resection margins () with 9 patients receiving adjuvant (salvage) radiotherapy and androgen deprivation therapy (ADT) (30.0%) after the surgery. Patients without positive resection margins did not receive adjuvant radiotherapy and/or ADT at any time before BCR. Follow-up was conducted in July 2021 through medical records. Attained follow-up data were time to BCR, death, time of death, and whether the cause of death was PCa related.
Table 1. Characteristics of radical prostatectomy (RP) specimens in the 68Ga-PSMA PET/CT cohort and in the 99mTc bone scintigraphy and CT-TAP cohort.
Imaging protocol
All 68Ga-PSMA PET/CT scans were performed approximately 60 min following intravenous injection of 2.14 MBq 68Ga-PSMA (68Ga-Glu-CO-Lys(Ahx)-HBED-CC) per kilogram body weight with low-dose CT for anatomical localization and attenuation correction. All patients were scanned on a Siemens Biograph TruePoint PET/CT scanner (Siemens, Erlangen, Germany). Images were reconstructed with all existing corrections applied (attenuation, scatter and Point-Spread Function) using the TrueX reconstruction algorithm (4 iterations and 21 subsets) and a 3 mm Gaussian post-filter (XYZ) and voxel size 2 × 2 × 2 mm.
Image analysis
All scans were analyzed by experienced, board certified specialists in nuclear medicine with common pitfalls in 68Ga-PSMA PET/CT in mind [Citation10]. Image analysis was performed using Hybrid Viewer (HERMES Medical Solutions AB, Stockholm, Sweden). Images were interpreted according to the PSMA-RADS Version 1.0-criteria [Citation11]. Maximum standardized uptake values (SUVmax) were measured in primary prostate tumors.
All 68Ga-PSMA PET/CT scans with extraprostatic extension or metastasis were discussed at multidisciplinary team conferences with board-certified oncologists, radiologists, urologists, pathologists, and nuclear medicine physicians. Only patients with localized disease in the prostate (cT1-cT3N0M0) or patients with oligometastatic disease (cT1-cT3N1M0) following primary staging by 68Ga-PSMA PET/CT, where the suspected regional lymph node metastases were deemed readily accessible for pelvic lymph node dissection, were scheduled for RP. All RP procedures were performed as robot-assisted surgery at the Department of Urology, Aarhus University Hospital, Denmark or the Department of Urology, Holstebro Regional Hospital, Denmark.
Data analysis
All statistical analyses were performed using R version 4.0.2 (R Foundation, Vienna, Austria) [Citation12]. The prognostic potential of 68Ga-PSMA PET/CT compared with conventional imaging was assessed by Cox regression providing hazard ratios (HRs) as well as Kaplan-Meier analysis and two-sided log-rank test using biochemical recurrence (BCR, defined as having a PSA value >0.2 ng/mL following RP) as clinical endpoint. All variables were summarized using descriptive statistics; values without normal distribution were described as median (range). Names of utilized statistical tests are provided in the results section. In all cases, p values < .05 were considered statistically significant.
External database and cohort
To compare the prognostic value of 68Ga-PSMA PET/CT with conventional imaging, we utilized an external cohort of RP patients from the Department of Urology, Aarhus University Hospital, Denmark. The cohort held 2,576 RP patients from May 2001 until January 2021. In total, 357 patients were registered as primary staged by 99mTc bone scintigraphy and CT-TAP with available follow-up and BCR status. After individual assessment, 137 patients were confirmed D’Amico high-risk individuals.
The radical prostatectomy procedure has largely been the same for many years at the facility, however in August 2005 the procedure was changed to be a robot-assisted procedure. The outcome for the patients has roughly been the same in terms of radical surgery, but with a decreased risk of erectile dysfunction and incontinence for robot surgery. A total of 34 patients (24.8%) experienced BCR after positive resection margins () with 4 patients receiving adjuvant (salvage) radiotherapy (11.8%). No patients received ADT after having positive resections margins. Patients without positive resection margins did not receive adjuvant radiotherapy and/or ADT at any time before BCR.
Results
Patient characteristics and biochemical recurrence
Of all 247 patients included in the 68Ga-PSMA PET/CT-cohort, 63 patients (25.5%) had BCR, defined as having a PSA value >0.2 ng/mL following RP within the follow-up period, while 184 patients (74.5%) remained free of recurrence after 5 years. The median follow-up time was 45.5 months (27.6–62.7 months) and median time to BCR was 13.2 months (1.9–55.2 months). For the conventional imaging cohort with 137 individuals, 69 patients (50.4%) remained free of recurrence after 5 years. Median follow-up time was 100.3 months (6.0–231.5 months) and median time to BCR was 18.6 months (1.1–116.9 months). The clinicopathological and RP characteristics of patients from both cohorts are summarized in and .
Table 2. Baseline and clinicopathological characteristics of the 68Ga-PSMA PET/CT cohort and the 99mTc bone scintigraphy and CT-TAP cohort.
Prognostic value of 68Ga-PSMA PET/CT for primary staging
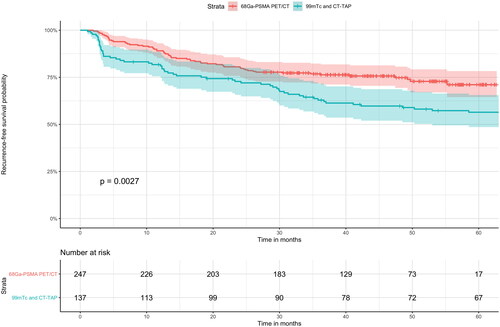
To assess the prognostic value of 68Ga-PSMA PET/CT for primary staging purposes, we compared 68Ga-PSMA PET/CT with 99mTc bone scintigraphy and CT-TAP as conventional imaging. Primary staging by 68Ga-PSMA PET/CT was significantly associated with longer time to BCR in univariate Cox regression analysis (HR = 0.58 (0.41–0.83), p = .004) compared with 99mTc bone scintigraphy and CT-TAP. Consistent with this, Kaplan-Meier analysis showed a better recurrence-free survival (RFS) probability (two-sided log-rank test, p = .003) (). The biochemical 5-year RFS rate was 71.1% (0.95 CI 64.5%-78.3%) in the 68Ga-PSMA PET/CT cohort compared with a 5-year RFS rate of 56.4% (0.95 CI 48.6–65.6%) in the 99mTc bone scintigraphy and CT-TAP cohort, thus yielding a 26.1% improvement with 68Ga-PSMA PET/CT (p = .003).
Figure 2. 68Ga-PSMA PET/CT compared with conventional imaging in relation to recurrence-free survival (RFS) in Kaplan-Meier curve analysis with 0.95 CI. Primary staging with 68Ga-PSMA PET/CT was significantly associated with a longer 5-year RFS rate compared with conventional imaging. The median duration of follow-up was 45.5 months in the 68Ga-PSMA PET/CT cohort and 100.3 months in the 99mTc bone scintigraphy and CT-TAP (thorax, abdomen, pelvis) group. The follow-up data of the conventional imaging cohort is censored at 60 months due to much longer follow-up time compared with the 68Ga-PSMA PET/CT cohort to improve visualization.
Discussion
In the present study, we found a lower recurrence rate after RP in the group primary staged with 68Ga-PSMA PET/CT compared with the conventional imaging group. To our knowledge, this is the first study to investigate the prognostic value of 68Ga-PSMA PET/CT compared with conventional imaging for primary staging in high-risk PCa patients treated with curative-intent RP. In comparison, the proPSMA trial demonstrated superior accuracy of 68Ga-PSMA PET/CT compared to conventional imaging [Citation3], and a study by Pyka et al. [Citation13] compared 68Ga-PSMA PET/CT and 99mTc bone scintigraphy for skeletal staging in primary PCa finding far superior region-based sensitivity (98.8–99.0%) and specificity (98.9–100%) of 68Ga-PSMA PET/CT compared with values of 99mTc bone scintigraphy (82.4–86.6% and 91.6–97.9%, respectively). In terms of lymph node involvement determined by CT scans, 68Ga-PSMA PET/CT caused considerable changes in N status with a 20% increase in level of confidence in a study by Donswijk et al. [Citation14].
Previous reports of D’Amico high-risk patients had a 5-year RFS rate of 58.4% [Citation4] very much in line with the present conventional imaging cohort. Based on the results from the present study, 68Ga-PSMA PET/CT may be recommended for primary staging prior to RP compared with conventional imaging due to higher diagnostic value and better selection of patients for surgery. The main study limitation was the comparability of the two cohorts used in the study which were not recruited in the exact same period and not completely matched. However, the frequency of adjuvant radiotherapy and/or ADT following positive resection margins was comparable as well as the surgical technique used throughout the years. Though, the positive resection margin rate was marginally higher in the external cohort, these major predictors of recurrence do not clearly explain the difference between the groups. The addition of adjuvant radiotherapy as well as ADT after a positive resection margin has only been implemented as standard in Denmark recently which explains the marginally higher percentage of additional therapy in the 68Ga-PSMA PET/CT cohort. Besides, this is a retrospective study design and our 68Ga-PSMA PET/CT cohort has shorter follow-up time compared with the conventional imaging cohort due to the relatively recent introduction of 68Ga-PSMA PET/CT for primary staging. However, the recurrence rate after RP following conventional imaging in the present study seems in line with previous published data [Citation4,Citation15,Citation16], and hence we consider it fair to conclude that our data could indicate a lower recurrence rate after RP following primary staging with 68Ga-PSMA PET/CT compared to conventional imaging. This would probably be caused by an improved selection of patients for surgery.
However, we do not know if the improved staging has an impact on PCa-specific or overall survival, as improved primary staging will eventually lead to stage migration (the Will Rogers phenomenon [Citation17]) with the movement of supposed former localized PCa patients, when staged by conventional imaging, to the oligometastatic group when staged by 68Ga-PSMA PET/CT. This will lead to fewer patients with true advanced disease in the RP group, but instead they will be added to the metastatic group despite having a very low metastatic burden. This will result in better survival in both above-mentioned groups. Unfortunately, PC-specific survival analyses could not be carried out due to insufficient follow-up time in our 68Ga-PSMA PET/CT cohort in a prostate cancer context.
Therefore, additional prospective studies of larger scale are warranted to confirm the results presented and to study the potential survival benefits of the entire cohort of PCa patients.
Conclusion
The present data could indicate a lower recurrence rate after RP following primary staging with 68Ga-PSMA PET/CT compared to conventional imaging, likely due to improved selection of patients for surgery. However, further prospective data on this topic are warranted.
Institutional review board statement
The Central Denmark Region Committees on Health Research Ethics (case number 1-10-72-361-18) and The Danish Data Protection Agency (case number 1-16-02-23-19) approved this retrospective study. The study was registered on the internal list of research projects in the Central Denmark Region.
Informed consent statement
The requirement to obtain informed consent was waived by The Central Denmark Region Committees on Health Research Ethics due to the retrospective study design.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Most data supporting the reported results are provided in the paper and further data are stored by the authors.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Pomykala KL, Farolfi A, Hadaschik B, et al. Molecular imaging for primary staging of prostate cancer. Semin Nucl Med. 2019;49(4):271–279.
- Hofman MS, Lawrentschuk N, Francis RJ, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet. 2020;395(10231):1208–1216.
- Murata Y, Tatsugami K, Yoshikawa M, et al. Predictive factors of biochemical recurrence after radical prostatectomy for high-risk prostate cancer. Int J Urol. 2018;25(3):284–289.
- D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974.
- Klingenberg S, Jochumsen MR, Ulhøi BP, et al. Ga-PSMA PET/CT for primary lymph node and distant metastasis NM staging of High-Risk prostate cancer. J Nucl Med. 2021;62(2):214–220.
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381.
- Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71(4):618–629.
- Briganti A, Chun FK-H, Salonia A, et al. Validation of a nomogram predicting the probability of lymph node invasion based on the extent of pelvic lymphadenectomy in patients with clinically localized prostate cancer. BJU Int. 2006;98(4):788–793.
- Sheikhbahaei S, Werner RA, Solnes LB, et al. Prostate-specific membrane antigen (PSMA)-targeted PET imaging of prostate cancer: an update on important pitfalls. Semin Nucl Med. 2019;49(4):255–270.
- Rowe SP, Pienta KJ, Pomper MG, et al. PSMA-RADS version 1.0: a step towards standardizing the interpretation and reporting of PSMA-targeted PET imaging studies. Eur Urol. 2018;73(4):485–487.
- R Core Team. R foundation for statistical computing. Vienna AR: A language and environment for statistical computing; 2019.
- Pyka T, Okamoto S, Dahlbender M, et al. Comparison of bone scintigraphy and (68)Ga-PSMA PET for skeletal staging in prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43(12):2114–2121.
- Donswijk ML, van Leeuwen PJ, Vegt E, et al. Clinical impact of PSMA PET/CT in primary prostate cancer compared to conventional nodal and distant staging: a retrospective single center study. BMC Cancer. 2020;20(1):723.
- Yuh B, Artibani W, Heidenreich A, et al. The role of robot-assisted radical prostatectomy and pelvic lymph node dissection in the management of high-risk prostate cancer: a systematic review. Eur Urol. 2014;65(5):918–927.
- Kamitani R, Matsumoto K, Kosaka T, et al. Evaluation of gleason grade group 5 in a contemporary prostate cancer grading system and literature review. Clin Genitourin Cancer. 2021;19(1):69–75.e5.
- Feinstein AR, Sosin DM, Wells CK. The will rogers phenomenon. Stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N Engl J Med. 1985;312(25):1604–1608.