Abstract
Background: Despite therapeutic progress, 10 to 30% of adult patients with primary mediastinal B cell lymphoma (PMBCL) are primary refractory or experience early relapse (R/R). Allogeneic stem cell transplantation (allo-HSCT) thus remains a potentially curative option in this setting.
Material and Methods: In this multicenter retrospective study, the outcomes of 33 French and Belgian adult patients allo-transplanted for R/R PMBCL between January 1999 and December 2018, were examined.
Results: At allo-HSCT time, patients had received a median of 3 treatment lines, 50% of them were in complete response, 40% in partial response and 10% had a progressive disease. Forty-two percent of the donors were siblings and 39% matched related. The median follow-up for alive patients was 78 months (3.5–157). Considering the whole cohort, 2-year overall survival (OS), progression free survival (PFS) and graft-versus-host disease-free/relapse-free survival (GRFS) were 48% (95%CI: 33–70), 47% (95%CI: 33–68) and 38.5% (95%CI: 25–60) respectively. Cumulative incidence of relapse and non-relapse mortality rates were respectively 34% (95%CI: 18–50) and 18% (95%CI: 7–34). Disease status at transplant was the only factor predicting survivals, patients with progressive disease showing significant lower 2-year PFS (HR: 6.12, 95%CI: 1.32–28.31, p = 0.02) and OS (HR: 7.04, 95%CI: 1.52–32.75, p = 0.013). A plateau was observed for OS and PFS after 4 years with 10 patients alive after this date, suggesting that almost one third of the patients effectively salvaged and undergoing allo-SCT could be cured.
Conclusion: This study indicates that allo-HSCT is a valid therapeutic option for R/R PMBCL, providing durable remissions.
Background
Primary mediastinal B-cell lymphoma (PMBCL) is identified as a unique and rare subtype of aggressive B-cell non Hodgkin lymphoma entity by the WHO classification, with distinct pathological, immunohistochemical, genetic and clinical features [Citation1–3]. The characteristics of PMBCL overlap with those of classic diffuse large B-cell lymphoma (DLBCL) and Hodgkin’s lymphoma [Citation4].
Because PMBCL is a rare condition, most data supporting clinical management have been extrapolated from retrospective series or subgroup analyses from prospective trials designed for DLBCL. Poly-chemotherapy with anthracyclines and anti-CD20 is the first-line standard-of-care for PMBCL [Citation5–10]. Despite the therapeutic progress brought by the frontline use of rituximab, 10 to 30% of patients with PMBCL are primary refractory or experience early relapse [Citation11,Citation12]. The historical second-line treatment of these patients was based on the use of intensive bridging chemotherapy with or without radiotherapy (RT), before autologous hematopoietic stem cell transplantation (auto-HSCT). However, the outcomes of this strategy remain largely unsatisfactory [Citation13–15].
New therapies such as chimeric antigen receptor T-cells (CAR T-cells) or programmed cell death 1 (PD-1) inhibitors alone or in association with brentuximab-vedotin, have shown encouraging results in relapse/refractory (R/R) PMBCL [Citation16–19]. Although these treatments can provide complete remission, most of the patients still experience a relapse.
Allogeneic HSCT (allo-HSCT) is a potentially curative treatment for patients with aggressive lymphoma relapsing after auto-HSCT [Citation20–23]. However, only few data are available in the specific setting of PMBCL. To our knowledge, the only large series, including 28 patients from 3 American centers, has been recently published by Herrera et al., with the encouraging results of 45% 5-year overall survival (OS) and 34% 5-year disease free survival (DFS) [Citation24].
To better appreciate the role of allo-HSCT for R/R PMBCL patients, in the context of emerging new immunotherapies, the outcomes of French and Belgian adult patients with R/R PMBCL, who received an allo-HSCT, were evaluated on behalf of the Société Francophone de Greffe de Moelle et de Thérapie Cellulaire (SFGM-TC) and the lymphoma study association (LYSA) group.
Material and methods
Eligibility criteria and study design
This multicenter retrospective study included all adult patients (≥18 years old) reported to the SFGM-TC registry who underwent an allo-HSCT for a PMBCL between January 2005 and December 2018 in France and Belgium. Data were obtained through the ProMISe database (an internet-based system shared by all European transplantation centers) and completed by consulting the medical files of patients from centers of the LYSA group. All patients provided informed consent for anonymously collecting their personal data in the ProMISe database. Histological diagnosis was performed at each center before transplantation, without centralized review. Response assessment was evaluated according to Cheson 2007 revised response criteria for malignant lymphoma up to 2014 [Citation25], then the Lugano classification, taken into account PET-CT results, was applied [Citation26,Citation27
].
Statistical analyses
The endpoints examined in this study were 2-year OS, progression-free survival (PFS), non-relapse mortality (NRM), graft versus host disease (GVHD)-free/relapse-free survival (GRFS) and cumulative incidence of relapse (CIR). OS was defined as the time from day 0 of allo-HSCT to death or last follow-up for survivors. PFS was defined as the time from day 0 of allo-HSCT to relapse or disease progression or death from any cause. NRM was defined as death from any cause without previous relapse or progression. GRFS was defined as alive patients with no documentation of serious GVHD (grade III-IV acute GVHD (aGVHD), moderate or severe chronic GVHD (cGVHD)) nor relapse or death. Relapse was defined as any event related to re-occurrence of the disease. OS and PFS curves were generated using the Kaplan-Meier method and compared using the Cox regression model. Cumulative incidence functions (CIF) were used to estimate CIR and NRM in a competing risk setting, differences among groups were calculated by Gray test. To study aGVHD and cGVHD, death and relapse were considered as competing events. aGVHD and cGVHD were staged according to standard criteria [Citation28,Citation29]. Survival probabilities are presented as percentages and 95% confidence intervals (95% CI).
Univariate analyses were carried out using the log-rank test for OS, PFS, NRM and GRFS and the Gray test for comparison of CIR. Characteristics considered for univariate analysis were: age at diagnosis (continuous variable), Ann Arbor stage (1–2 vs 3–4), LDH levels (normal vs elevated), IPI score (1–2 versus 3–4), primary refractoriness (no vs yes), number of lines of treatment before transplant (continuous variable), disease status at transplant (complete response, (CR) or partial response (PR), progressive disease, (PD)), number of responses before transplant (1 vs 2 vs 3), age at transplant (continuous variable), HCT-CI score (<3 vs > =3), Karnofsky score at transplant (continuous variable), graft number (auto and allo-HSCT), myeloablative conditioning regimen (MAC) (no vs yes), type of donor (identical sibling, haploidentical sibling, matched unrelated, mismatched unrelated, umbilical cord blood), ABO matching (compatible, major incompatibility, minor incompatibility), donor sex (female vs male), donor age (continuous variable), donor CMV status (negative vs positive), stem cell source (peripheral blood stem cells, PBSC, bone marrow, cord blood unit), GVHD prophylaxis (calcineurin inhibitor (CI) alone, CI + methotrexate (MTX), CI + mycophenolate mofetil (MMF), CI + MMF + post-transplant cyclophosphamide (PT-Cy), MTX + PT-Cy), engraftment (yes vs no) [Citation30,Citation31]. Due to the limited number of patients, adjustments for multivariate analysis were not possible here. All tests were two-sided and P values <0.05 were considered statistically significant. Analyses were carried out using the R core team (2021) statistical software version 4.1.1 (https://www.R-project.org/.).
Results
The cohort included 33 patients with PMBCL from 19 French (n = 29) and 3 Belgian (n = 4) centers. Patients and allo-HSCT characteristics are described in . The median age at transplant was 33 years old (range 18–61), with a predominance of female patients (58%, n = 19). At diagnosis, 76% of the patients had an IPI score at 1 or 2 and the Ann Arbor stage was 3–4 in 56.6% of them.
Table 1. Patients, disease and transplant characteristics.
All patients received poly-chemotherapy with anthracyclines and anti-CD20 as first-line therapy. Forty-one percent of the patients were refractory to this first line (n = 11, missing data: 6) but all had received a salvage chemotherapy thereafter. Median number of previous lines of treatment was 3 (1–6), including auto-HSCT in 20 patients (61%). Three and 2 patients had also received an anti-PD1 antibody and brentuximab vedotin before allo-HSCT, respectively.
At the time of transplant, 50% of the patients were in CR (n = 15), 40% in PR (n = 12) and 10% had a PD (n = 3) (missing data: 3). The majority of patients had an HCT-CI score <3 (n = 11/17, (64.7%), missing data: 16). Conditioning regimen were of reduced intensity (RIC) in 63% of the cases. Only one patient, in PR before allo-HSCT, received a sequential conditioning. The stem cell source was PBSC in 88% of the cases. Donors were siblings in 42% (n = 14) of the cases, matched unrelated in 39% (n = 13), mismatched unrelated in 9% (n = 3), haplo-identical in 6% (n = 2) and umbilical cord blood in 3% (n = 1).
Median follow up was 78 months (3.5–157). Primary graft failure occurred in one case. The median time until neutrophil and platelet recovery was respectively d18 (range 5–27) and d15 (range 10–46). Cumulative incidences of day 100 grade I-II and III-IV aGVHD were 36% and 0%, respectively. Cumulative incidence of cGVHD was 32% including one third of extensive cGVHD ().
Table 2. Patient outcomes.
Considering the whole cohort, the overall OS, PFS, NRM, GRFS and CIR at 2 years were 48% (95%CI: 33–70), 47% (95%CI: 33–68), 18% (95%CI: 7–34), 38.5% (95% CI: 25–60), and 34% (95%CI: 18–50) respectively (, ). 5-year OS and PFS were respectively 44% (95%CI: 29–67%) and 43% (95%CI: 29–65%). The median time between transplant and relapse was 75 days (13–272). A plateau was observed for OS and PFS after 4 years with 10 patients alive after this date.
Figure 1. Two year outcomes: a) 2 year PFS, b) 2 years OS, c) 2 year NRM, d) 2 year GRFS. GRFS: GVHD free relapse free survival; NRM: non relapse mortality; OS: overall survival; PFS: progression free survival
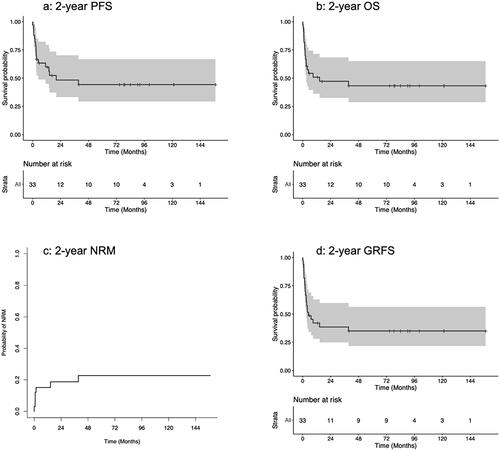
Patients with progressive disease at transplantation had worse PFS, OS and GRFS at 2 years (PFS: HR: 6.12, 95%CI: 1.32–28.31, p = 0.02, OS: HR: 7.04, 95%CI: 1.52–32.75, p = 0.013, GRFS: HR: 7.04, 95%CI: 1.52–32.75, p = 0.013). Patients who did not respond to the first line immunochemotherapy had similar OS and PFS compared to others. Conditioning intensity did not have any significant impact on OS and PFS.
Discussion
This retrospective multicenter study allowed to evaluate outcomes in a consistent cohort of 33 patients with the rare condition of R/R PMBCL who benefited from allo-HSCT. The results are very encouraging results with a 5-year OS and PFS of 44% and 43% respectively in these heavily pretreated patients, with acceptable toxicity. This relatively low toxicity is mostly due to the young age of the transplanted patients and their good performance status at transplantation. Of high interest, the post-allograft responses appear to be prolonged with an OS and PFS at 5 years at respectively 44% (95%CI 29–67%) and 43% (95%CI 29–65%), suggesting a potential cure in almost one third of the patients.
The therapeutic landscape of PMBCL has recently evolved with the growing importance of immunotherapy such as anti-PD1 alone or in association with brentuximab vedotin [Citation16,Citation32–34]. As well, the development of CAR T-cells appears to be an important paradigm shift in the management of B-cells malignancies [Citation17,Citation33,Citation34]. Anti-CD19 CAR T-cells have been approved by the FDA for aggressive R/R non Hodgkin lymphomas (NHL), including PMBCL. However, only few patients with PMBCL were included in the pilot studies ZUMA-1 and TRANSCEND-NHL-001(17) [Citation34,Citation35]. Crombie et al recently reported the real-life results of 33 patients treated with axicabtagene ciloleucel for R/R PMBCL. The overall response rate was 78% with 69% of CR. The 24-month (intent-to-treat) PFS was 64% and 24-month OS 78% [Citation19], which appears to be superior to the results reported in our cohort.
The role of allo-HSCT in the therapeutic strategy of R/R PMBCL in the CAR T-cells era remains unclear in the absence of comparative data. In the setting of R/R DLBCL, due to high response rates and low short and long term toxicity, currently available data suggest that CAR T-cells should be preferred over allo-HSCT when available [Citation36,Citation37]. The preference for allo-HSCT appears to be limited to situations where CAR T-cells are deemed not feasible or not useful, such as patients with refractory cytopenias or incipient myelodysplastic syndrome [Citation38]. Allo-HSCT probably remains an interesting option in case of post CAR-T-cells relapse but requires effective salvage bridging therapy. In addition, the question of the place of allo-HSCT as consolidation therapy after CAR T-cells will have to be evaluated prospectively [Citation39]. In our cohort, only one patient received CAR T-cells prior to allo-HSCT, achieving a PR and then receiving haplo-identical HSCT. This sequence resulted in a durable complete remission, and the patient was still in CR at the last follow-up visit.
In the cohort reported here, the type of conditioning intensity (MAC or RIC) did not have any significant impact on OS nor PFS. These results are consistent with literature data regarding allo-HSCT for R/R DLBCL [Citation20,Citation22]. Therefore, it does not seem appropriate to propose myeloablative conditioning in these already heavily pretreated patients.
Compared with the series of Herrera et al., OS and PFS at 2 years are similar, but the rate of NRM is lower in our study (18 vs 29%) [Citation24]. This difference is difficult to explain, as our series contained more patients receiving myeloablative conditioning (14 vs 33%). By contrast, primary refractory patients appeared to benefit from allo-HSCT if they could achieve at least a PR with bridging therapy in our cohort.
Haploidentical transplant with post-infusion cyclophosphamide (haplo-HSCT) has been widely developed over the past decade. Although no specific data are published for PMBCL, retrospective studies suggest that outcomes after haplo-HSCT are similar to non-haplo-HSCT in NHL [Citation40–42]. In contrast to Hodgkin’s lymphoma, there is no evidence in favor of haplo-SCT over HLA-matched donor for NHL [Citation39]. Haplo-HSCT is currently recommended by the EMBT in the treatment of high-grade lymphoma as a safe option for patients without an HLA-matched donor. In our cohort, no influence of the donor type was observed on post-allograft outcomes.
The timing of the allograft remains another difficult question. Our results show that allo-HSCT should not be considered for patients who do not respond to salvage strategies. A sequential approach combining chemotherapy with broad antitumor activity followed by a reduced-intensity conditioning regimen has been shown to be useful in refractory AML and anecdotally in other hematological malignancies [Citation40,Citation43–45]. Prophylaxis of post-transplant relapse by donor lymphocytes infusion (DLI) has also been shown to be useful in high-risk myeloid hematological malignancies [Citation46,Citation47]. A trial is currently ongoing to evaluate the interest of performing an allograft after sequential conditioning followed by DLI in R/R lymphoid malignancies (NCT03079089).
Although this study is retrospective series, it has included a significant number of patients suffering from this rare condition. It provided encouraging results suggesting that allo-HSCT should be considered in patients with R/R PMBCL who achieve at least a PR after salvage therapy. Prospective trials are needed to determine the interest and timing of allo-HSCT in the context of emerging promising immunotherapy.
Author contributions
A.L.B, B.L.C, S.L.G and P.C designed, performed, coordinated the research, analyzed, interpreted the data, and wrote the manuscript. B.T performed the statistical analyses. L.R, C.M.G, M.S, G.G, A.F, R.G, S.N.Q, S.F, A.C, D.S, P.L, X.P, N.M, A.V, M.L, J.P, Y.B, R.D, J.T, V.C, M-C.B contributed data and commented on the manuscript.
Acknowledgments
The authors thank all investigators and data managers for their dedicated patient care.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, Amandine Le Bourgeois, upon reasonable request.
References
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the world health organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390.
- Savage KJ, Monti S, Kutok JL, et al. The molecular signature of mediastinal large B-cell lymphoma differs from that of other diffuse large B-cell lymphomas and shares features with classical hodgkin lymphoma. Blood. 2003;102(12):3871–3879.
- Abou-Elella AA, Weisenburger DD, Vose JM, et al. Primary mediastinal large B-Cell lymphoma: a clinicopathologic study of 43 patients from the Nebraska lymphoma study group. J Clin Oncol. 1999;17(3):784–90790.
- Giulino-Roth L. How I treat primary mediastinal B-cell lymphoma. Blood. 2018;132(8):782–790.
- Vitolo U, Seymour JF, Martelli M, et al. Extranodal diffuse large B-cell lymphoma (DLBCL) and primary mediastinal B-cell lymphoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:91–102.
- Cwynarski K, Marzolini MAV, Barrington SF, et al.; British Society for Haematology Guidelines. The management of primary mediastinal B‐cell lymphoma: a british society for haematology good practice paper. Br J Haematol. 2019;185(3):402–409.
- Camus V, Rossi C, Sesques P, et al. Outcomes after first-line immunochemotherapy for primary mediastinal B-cell lymphoma: a LYSA study. Blood Adv. 2021;5(19):3862–3872.
- Wästerlid T, Hasselblom S, Joelsson J, et al. Real-world data on treatment and outcomes of patients with primary mediastinal large B-cell lymphoma: a swedish lymphoma register study. Blood Cancer J. 2021;11(5):100.
- Dunleavy K, Pittaluga S, Maeda LS, et al. Dose-Adjusted EPOCH-Rituximab therapy in primary mediastinal B-Cell lymphoma. N Engl J Med. 2013;368(15):1408–1416.
- Eule C, Arora N, Hsiao C, et al. Presentation and management of primary mediastinal large B-cell lymphoma: a retrospective cohort analysis. Acta Oncol. 2020;59(7):786–788.
- Giulino‐Roth L, O’Donohue T, Chen Z, et al. Outcomes of adults and children with primary mediastinal B-cell lymphoma treated with dose-adjusted EPOCH-R. Br J Haematol. 2017;179(5):739–747.
- Soumerai JD, Hellmann MD, Feng Y, et al. Treatment of primary mediastinal B-cell lymphoma with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone is associated with a high rate of primary refractory disease. Leuk Lymphoma. 2014;55(3):538–543.
- Aoki T, Shimada K, Suzuki R, et al. High-dose chemotherapy followed by autologous stem cell transplantation for relapsed/refractory primary mediastinal large B-cell lymphoma. Blood Cancer J. 2015;5(12):e372-e372.
- Kuruvilla J, Pintilie M, Tsang R, et al. Salvage chemotherapy and autologous stem cell transplantation are inferior for relapsed or refractory primary mediastinal large B-cell lymphoma compared with diffuse large B-cell lymphoma. Leuk Lymphoma. 2008;49(7):1329–1336.
- Vardhana S, Hamlin PA, Yang J, et al. Outcomes of relapsed and refractory primary mediastinal (thymic) large B cell lymphoma treated with Second-Line therapy and intent to transplant. Biol Blood Marrow Transplant. 2018;24(10):2133–2138.
- Armand P, Rodig S, Melnichenko V, et al. Pembrolizumab in relapsed or refractory primary mediastinal large B-Cell lymphoma. J Clin Oncol. 2019;37(34):3291–3299.
- Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-Cell therapy in refractory large B-Cell lymphoma. N Engl J Med. 2017;377(26):2531–2544.
- Zinzani PL, Santoro A, Gritti G, et al. Nivolumab combined with brentuximab vedotin for relapsed/refractory primary mediastinal large B-Cell lymphoma: Efficacy and safety from the phase II CheckMate 436 study. J Clin Oncol. 2019;37(33):3081–3089.
- Crombie JL, Nastoupil LJ, Redd R, et al. Real-world outcomes of axicabtagene ciloleucel in adult patients with primary mediastinal B-cell lymphoma. Blood Adv. 2021;5(18):3563–3567.
- Fenske TS, Ahn KW, Graff TM, et al. Allogeneic transplantation provides durable remission in a subset of DLBCL patients relapsing after autologous transplantation. Br J Haematol. 2016;174(2):235–248.
- Bacher U, Klyuchnikov E, Le-Rademacher J, et al. Conditioning regimens for allotransplants for diffuse large B-cell lymphoma: myeloablative or reduced intensity? Blood. 2012;120(20):4256–4262.
- Glass B, Hasenkamp J, Wulf G, et al. Rituximab after lymphoma-directed conditioning and allogeneic stem-cell transplantation for relapsed and refractory aggressive non-Hodgkin lymphoma (DSHNHL R3): an open-label, randomised, phase 2 trial. Lancet Oncol. 2014;15(7):757–766.
- Le Bourgeois A, Labopin M, Blaise D, Société Francophone de Greffe de Moelle et de Thérapie Cellulaire, et al. Reduced-intensity versus reduced-toxicity myeloablative fludarabine/busulfan-based conditioning regimens for allografted non-Hodgkin lymphoma adult patients: a retrospective study on behalf of the. Ann Oncol. 2017;28(9):2191–2198.
- Herrera AF, Chen L, Khajavian S, et al. Allogeneic stem cell transplantation provides durable remission in patients with primary mediastinal large B cell lymphoma. Biol Blood Marrow Transplant. 2019;25(12):2383–2387.
- Cheson BD, Pfistner B, Juweid ME, International Harmonization Project on Lymphoma, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–586.
- Cheson BD, Fisher RI, Barrington SF, United Kingdom National Cancer Research Institute, et al. Recommendations for initial evaluation, staging, and response assessment of hodgkin and Non-Hodgkin lymphoma: the lugano classification. J Clin Oncol. 2014;32(27):3059–683068.
- Ruggeri A, Labopin M, Ciceri F, et al. Definition of GvHD-free, relapse-free survival for registry-based studies: an ALWP–EBMT analysis on patients with AML in remission. Bone Marrow Transplant. 2016;51(4):610–611.
- Harris AC, Young R, Devine S, et al. International, multicenter standardization of acute graft-versus-Host disease clinical data collection: a report from the mount sinai acute GVHD international consortium. Biol Blood Marrow Transplant. 2016;22(1):4–10.
- Martin PJ, Lee SJ, Przepiorka D, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-Host disease: VI. The 2014 clinical trial design working group report. Biol Blood Marrow Transplant. 2015;21(8):1343–1359.
- International Non-Hodgkin’s lymphoma prognostic factors project. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1993;329(14):987–994.
- Carbone PP, Kaplan HS, Musshoff K, et al. Report of the committee on hodgkin’s disease staging classification. Cancer Res. 1971;31(11):1860–1861.
- Zinzani PL, Ribrag V, Moskowitz CH, et al. Safety and tolerability of pembrolizumab in patients with relapsed/refractory primary mediastinal large B-cell lymphoma. Blood. 2017;130(3):267–270.
- Schuster SJ, Bishop MR, Tam CS, JULIET Investigators, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-Cell lymphoma. N Engl J Med. 2019;380(1):45–56.
- Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. The Lancet. 2020;396(10254):839–852.
- Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20(1):31–42.
- Hamadani M, Gopal AK, Pasquini M, et al. Allogeneic transplant and CAR-T therapy after autologous transplant failure in DLBCL: a noncomparative cohort analysis. Blood Adv. 2022;6(2):486–494.
- Shah NN, Hamadani M. Is there still a role for allogeneic transplantation in the management of lymphoma. J Clin Oncol. 2021;39(5):487–498.
- Dreger P, Fenske TS, Montoto S, European Society for Blood and Marrow Transplantation (EBMT) and the Center for International Blood and Marrow Transplant Research (CIBMTR), et al. Cellular immunotherapy for refractory diffuse large B cell lymphoma in the chimeric antigen receptor-engineered T cell era: still a role for allogeneic transplantation? Biol Blood Marrow Transplant. 2020;26(4):e77–e85.
- Shadman M, Gauthier J, Hay KA, et al. Safety of allogeneic hematopoietic cell transplant in adults after CD19-targeted CAR T-cell therapy. Blood Adv. 2019;3(20):3062–3069. 22
- Zoellner A-K, Fritsch S, Prevalsek D, et al. Sequential therapy combining clofarabine and T-cell-replete HLA-haploidentical haematopoietic SCT is feasible and shows efficacy in the treatment of refractory or relapsed aggressive lymphoma. Bone Marrow Transplant. 2015;50(5):679–684.
- Garciaz S, Castagna L, Bouabdallah R, et al. Familial haploidentical challenging unrelated donor Allo-SCT in advanced non-Hodgkin lymphomas when matched related donor is not available. Bone Marrow Transplant. 2015;50(6):865–867.
- Mariotti J, Devillier R, Bramanti S, et al. T Cell-Replete haploidentical transplantation with Post-Transplantation cyclophosphamide for hodgkin lymphoma relapsed after autologous transplantation: Reduced incidence of relapse and of chronic graft-versus-Host disease compared with HLA-Identical related donors. Biol Blood Marrow Transplant. 2018;24(3):627–632.
- Gauthier J, Poiré X, Gac A-C, et al. Better outcome with haploidentical over HLA-matched related donors in patients with hodgkin’s lymphoma undergoing allogeneic haematopoietic cell transplantation—a study by the francophone society of bone marrow transplantation and cellular therapy. Bone Marrow Transplant. 2018;53(4):400–409.
- Schmid C, Schleuning M, Schwerdtfeger R, et al. Long-term survival in refractory acute myeloid leukemia after sequential treatment with chemotherapy and reduced-intensity conditioning for allogeneic stem cell transplantation. Blood. 2006;108(3):1092–1099.
- Duléry R, Ménard A-L, Chantepie S, et al. Sequential conditioning with thiotepa in T Cell- Replete hematopoietic stem cell transplantation for the treatment of refractory hematologic malignancies: Comparison with matched related, Haplo-Mismatched, and unrelated donors. Biol Blood Marrow Transplant. 2018;24(5):1013–1021.
- Kolb H-J. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 2008;112(12):4371–4383.
- Guillaume T, Porcheron S, Audat F, et al. Recommandations de la SFGM-TC concernant l’injection prophylactique, préemptive et curative des lymphocytes du donneur (DLI) après allogreffe de cellules souches hématopoïétiques. Pathol Biol. 2014;62(4):193–196.
