Abstract
Background
Computed tomography (CT) examinations are increasingly used worldwide and incidental findings are growing likewise. Lung cancer stage at diagnosis is pivotal to survival. The earliest stage of lung cancer, stage IA is in most cases asymptomatic. Potentially, increased use of clinical CTs could induce a stage shift toward earlier lung cancer diagnosis.
Materials and methods
Data on the number of CT thorax in Denmark and the stage distribution of Danish lung cancer patients 2013–2020 were acquired from, respectively, the Danish Health Data Authority and the Danish Lung Cancer Registry. Clinical auditing of stage IA lung cancer patients was performed in the period 2019–2021 in a Danish region to assess the reasons for referral. Auditing of stage IV lung cancer patients was done to see whether a CT thorax was performed in a two-year period before diagnosis.
Results
All regions showed an increase in CTs per 1000 inhabitants. However, the number of CTs performed in 2013 differed by more than 50% among regions, and the increase per year also differed, from an increase of 1.9 to 3.4 more examinations per year. A significant correlation between CTs and fraction of stage IA lung cancers was seen in four out of the five regions. The audit of stage IA lung cancer cases revealed that 86.8% were incidental findings. Audit of stage IV lung cancer found that 4.3% had a nodule/infiltrate on a previous CT within a 2-year period prior to the diagnosis of lung cancer that was the probable origin of stage IV lung cancer.
Conclusion
The study found that the vast majority of early-stage lung cancers were incidental findings. It highlights that follow-up algorithms of incidental findings should be used in accordance with guidelines and it should be unequivocally how the CT follow-up of pulmonary infiltrates is managed.
Background
Globally, approximately 300 million computed tomography (CT) examinations are performed in a year and it is increasing by 4% per year [Citation1]. However, the use of CT examinations varies considerably in Western countries [Citation2]. The US is one of the countries that performs the most CT examinations (255 per 1000 inhabitants; 2021), while Finland is among the Western countries performing the fewest (45 per 1000 inhabitants; 2020). In Denmark, the annual number of CT examinations is relatively high, at 207 per 1000 inhabitants (2021) performed annually [Citation3], which probably reflects the number of available CT scanners. The incidence of lung cancer in Denmark is also high with an age-standardized incidence rate of 39.4 per 100,000 per year (men). This is considerable higher compared to the other Nordic countries (Norway: 32.5; Sweden 17.7; Finland 29.0 per 100,000 per year) [Citation4]. Among the Danish regions, the age-standardized incidence rate varies between 37.7 and 43.2 per 100,000 per year (men) [Citation4]. A lung cancer screening program has not been implemented in Denmark.
The frequency of incidental findings from CT examinations is around 31% [Citation5]. These include both incidental findings without clinical relevance and potentially malignant lesions. Hence, if more CT examinations of the thorax (CT thorax) are performed, patients with lung cancer might incidentally be diagnosed at an early stage. Incidentally detected lung tumors are typically smaller, found at an earlier stage and are more often adenocarcinomas. Incidental detection of a lung cancer has previously been shown to serve as an independent survival predictor, compared to symptomatic patients or x-ray-detected tumors [Citation6–9]. However, some incidentally detected tumors could be indolent and not influence mortality [Citation10].
In most cases, the earliest stage of lung cancer, stage IA, is asymptomatic and is best detected by CT. It was found in a single-center clinical audit of stage I lung cancer patients, that as many as 61% of their tumors were incidental findings [Citation11]. In this study, no difference in survival was seen between incidentally detected tumors vs. non-incidentally detected tumors, possibly because the symptoms in stage I lung cancer patients often do not stem from the actual tumor, but usually from comorbidity, such as chronic obstructive pulmonary disease, emphysema, or pulmonary infections that may have led to the CT examination revealing the tumor.
At present, it is not known whether the increase in clinical use of CT examinations is linked to a stage shift toward earlier lung cancer diagnosis. However, if the majority of stage I lung cancers detected are incidental findings on clinical CT examinations, then a logical consequence of an increased use of CT examinations would be the detection of an increased number of early-stage lung cancers. Furthermore, it becomes paramount to ensure a correct follow-up of incidental CT examinations findings of possible early-stage lung cancer on.
This study aimed to evaluate the development in number of clinical CT thorax examinations and the fraction of stage IA lung cancer cases over an 8-year period in Denmark. As part of this analysis, a clinical audit was performed in one of the regions, to assess how many of the stage IA patients were diagnosed as the result of an incidental finding, and whether some of the patients diagnosed with stage IV lung cancer could have been diagnosed earlier, if adequate follow-up of incidental findings had taken place.
Materials and methods
CT thorax examinations and lung cancer stage distribution
Denmark is divided in five regions. Data on the annual use of CT thorax examinations 2013–2020 in the five Danish regions were acquired from the Danish Health Data Authority. The stage distribution of lung cancer patients 2013–2020 in the five Danish regions was extracted from the Danish Lung Cancer Registry [Citation12]. The combination of the five Danish regions provides the national average.
Clinical audit of lung cancer patients diagnosed in stage IA
An audit of patient journals was performed in one of the regions. All lung cancer patients in clinical stage IA (TNM eighth edition [Citation13]) in the period 2019–2021 were identified from the Danish Lung Cancer Registry [Citation12]. Auditing was performed to investigate whether the reason for referral to the CT thorax examination was a clinical suspicion of lung cancer or any other reason (i.e., incidental finding). If the CT thorax examination was performed as a consequence of a chest x-ray, the reason for referral for chest x-ray was noted. Furthermore, it was stated whether the stage was IA1, IA2, or IA3 (i.e., tumor size 0–10 mm; 11–20 mm, or 21–30 mm).
Clinical audit of lung cancer patients diagnosed in stage IV
An audit of patient journals was performed in one of the regions. All lung cancer patients in stage IV 2019–2021 were identified from the Danish Lung Cancer Registry [Citation12]. Next, auditing was performed to evaluate if patients had had a CT examination that showed parts of the lungs within the two years before lung cancer diagnosis. The types of CT scans included were: CT thorax (including CT angiography), CT thorax abdomen pelvis (CT TAP), CT abdomen, showing lower parts of the lungs (including CT urography) and cardiac CT. If patients had a CT scan performed two years before the lung cancer diagnosis, it was noted whether the CT examination was described with a pulmonary nodule/infiltrate and if adequate follow-up with CT was performed. If the CT scan was not described with pulmonary nodules/infiltrates, the scan was assessed to evaluate, in retrospect, if any pulmonary nodules/infiltrates could be detected at the location where the lung tumor was found. Finally, it was assessed whether any pulmonary nodules/infiltrates seen on the earlier CT scan were likely to be the origin of the later diagnosed stage IV lung cancer.
Statistics
Correlations between number of CT examinations per 1000 inhabitants and lung cancer stage distribution as well as variations among regions were calculated using linear regression analysis. Data analyses were performed in STATA Statistical Software, College Station, TX: StataCorp LLC. Auditing was performed using REDCap [Citation14].
Ethics
Data on annual use of CT examinations and lung cancer stage distribution are publicly available. Permission to conduct auditing was given by the hospital directors under the regulations of a quality improvement study.
Results
CT examinations and lung cancer stage at diagnosis
The development in the annual number of CT thorax examinations per 1000 inhabitants in the Danish regions is presented separately by region and as the mean of regions in . All regions show an increase in the number of CT thorax per 1000 inhabitants in the period 2013–2020. However, there are some variations among the regions in the number of CT thorax. The number of CT thorax per 1000 inhabitants performed in 2013 differed by more than 50%, from 24.3 to 38.9 (p < 0.01), and the increase per year over the eight years from 2013 to 2020 also differed, from an increase of 1.9 more CTs per year to 3.4 more per year (p < 0.01). At the end of the period, the relative difference between the regions was somewhat reduced, as the Central Denmark Region and North Denmark Region were performing almost 60 CT thorax per 1000 inhabitants in 2020, while the Capital Region and Region Zealand were performing 45–50 CT thorax per 1000 inhabitants in 2020.
Figure 1. (A) Development of CT thorax per 1000 inhabitants 2013–2020. (B) Fraction of stage IA lung cancer 2013–2020.
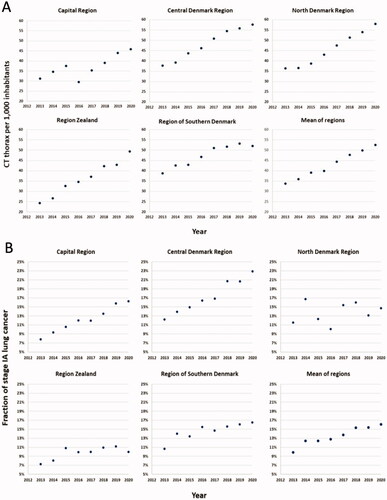
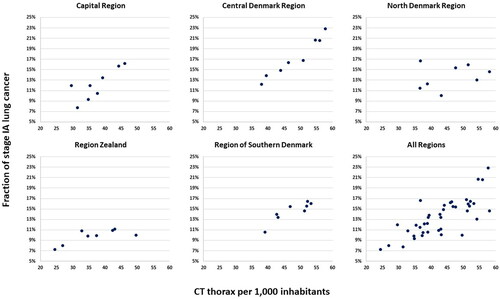
The fraction of stage IA lung cancer cases in Denmark and the five Danish regions in the period 2013–2020 is presented in . In 2013, the fraction of patients diagnosed in stage IA differed between regions, from 7.3% to 12.2% (p < 0.01). Over the following years, from 2013 to 2020, there were significant annual increases in the fraction of patients diagnosed in stage IA in the Capital Region, the Central Denmark Region, and the Region of Southern Denmark (p < 0.01 for each of these regions). In Region Zealand only a minor, but still significant, increase in stage IA fraction was seen (p < 0.05), while there was no significant change in the North Denmark Region.
A regression analysis of the correlation between the number of CT examinations and fraction of stage IA lung cancers was done. The scatter plots are presented in . In the Capital Region, Central Denmark Region, and Region of Southern Denmark, the fraction of patients diagnosed in stage IA correlated to approximately the same extent with the number of CT thorax examinations per 1000 inhabitants (p < 0.05 for the correlation between number of CT thorax examinations and the stage IA fraction, while p > 0.35 for difference between these three regions). A significant correlation was also seen in Region Zealand, although not nearly as marked (p < 0.05 for the correlation between number of CT thorax and the stage IA fraction, while p < 0.01 for the difference to the three other regions). In the North Denmark Region, there was no significant correlation between the number of CT examinations and fraction of stage IA lung cancer cases.
Clinical audit of lung cancer patients diagnosed in stage IA
In total, 278 patients with stage IA lung cancer were found. Five patients were excluded (three were not found to be stage IA and two referrals were not accessible), leaving 273 patients for auditing. Out of these, 36/273 (13.2%) were referred for suspicion of lung cancer, with seven (19.4%) in stage IA1, 23 (72.2%) in stage IA2 and 6 (16.7%) in stage IA3.
In total, 237/273 (86.8%) were incidental findings, with 38 (16.0%) in stage IA1, 151 (63.7%) in stage IA2 and 48 (20.3%) in stage IA3. The CT exams with incidental findings were performed as a result of trauma, shoulder pain, cardiac CT due to chest pain, suspicion of pulmonary embolism and investigation or follow-up of cancer in the colon, prostate, ovaries, and head and neck.
Clinical audit of lung cancer patients diagnosed in stage IV
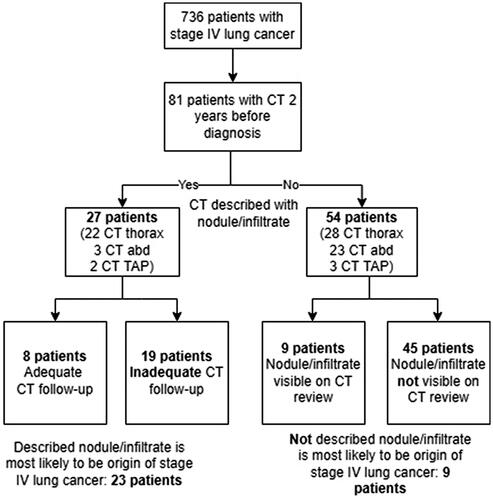
A total of 748 patients with stage IV lung cancer were found. After the exclusion of 12 patients (eleven duplicates and one with incorrect central person registration number format), 736 patients were audited. As shown in , 81 patients (11%) had had a clinical CT examination performed within a period of 2 years before the lung cancer diagnosis; 27 were described with a nodule/infiltrate by the radiologist, and of these, 19 patients did not receive adequate follow-up CT, in accordance with the Fleischner Society pulmonary nodules recommendations [Citation15]. Morphology, size and location of the 19 infiltrates/nodules are described in . Among the 27 patients with a pulmonary nodule/infiltrate previously described on CT, 23 of these nodules/infiltrates were likely to have developed into the subsequently diagnosed stage IV lung cancer, even though four of them received adequate follow-up CT.
Figure 4. Example of infiltrate in right upper lobe that did not receive follow-up. Computed tomography examinations taken 10 months apart. Diagnosed with T4N2M1c small cell carcinoma.
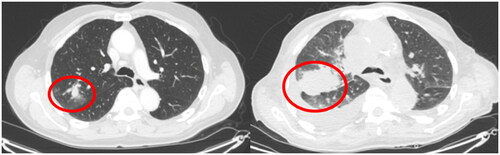
Table 1. Characteristics of nodules/infiltrates that did not receive follow-up.
Of the 81 patients with a previous CT examination two years before diagnosis, 54 were not described with a nodule/infiltrate. However, retrospectively assessed, nine patients did have a nodule/infiltrate on the CT examination that was likely to be the origin of the later stage IV lung cancer. So, in total, 32 out of 736 patients (4.3%) had a nodule/infiltrate on a previous CT within a 2-year period prior to the diagnosis of lung cancer that most likely developed into a stage IV lung cancer. An example of an infiltrate that developed into stage IV lung cancer from the clinical audit is provided in .
Discussion
This study examined the relationship between the annual use of CT thorax examinations and the fraction of stage IA lung cancer in the five Danish regions 2013–2020. The use of CT thorax examinations increased in all Danish regions, although the number of CT thorax examinations per 1000 inhabitants showed regional variations. Regional differences were also seen in the fraction of stage IA lung cancer and in the annually increase.
The clinical audit of patients with stage IA lung cancer showed that only 13.2% of referrals were on suspicion of lung cancer, while 86.8% were incidental findings.
The clinical audit of patients with stage IV lung cancer showed that 4.3% of patients had a CT examination performed within the previous 2-year period on which a nodule/infiltrate that likely developed into the stage IV lung cancer could be identified.
In line with the overall worldwide trend [Citation1], this study finds an increase in the clinical use of CT examinations in Denmark through the years 2013–2020. Positive correlations between the extent to which CT thorax examinations are used, and the fraction of stage IA lung cancer are also found in four of the five Danish regions. However, the fraction of stage IA lung cancer in the year 2020 shows wide variations among the Danish regions, with the highest stage IA fraction in the Central Denmark Region (22.9%) and the lowest in Region Zealand (10.0%). Previous US studies on differences in lung cancer stage distribution have found that insurance coverage has a great impact on stage at diagnosis and survival [Citation16], and that the military veterans covered by the Military Health System in the US have a higher percentage of stage I lung cancer, compared to the general population [Citation17]. However, compared to the US, the Danish health care system provides comprehensive care for all citizens, free of charge or with minimal costs. A study comparing the Danish regions also found substantial homogeneity regarding sociodemographic and health-related characteristics [Citation18]. Thus, the differences seen among regions could potentially stem from differences in hospital management of incidental findings from CT thorax. Another possible reason could be the scarcity of radiologists in rural parts of Denmark. In the Capital Region, the number of radiologists per 100,000 inhabitants is 13.8. Contrary, in Region Zealand, there are 6.9 radiologists per 100,000 inhabitants (2019). Second reads are not used in any of the Danish Regions.
Several low-dose CT lung cancer screening studies have shown a reduced lung cancer-specific mortality [Citation19–21]. However, the effect of the increased clinical use of CT examinations for many reasons other than suspicion of lung cancer (i.e., CT abdomen with lower lung segments, cardiac CT, etc.) on the stage distribution of diagnosed lung cancer has not been investigated previously. This study finds positive correlations between the intensity of clinical use of CT thorax examinations and the fraction of stage IA lung cancer stage. Clearly, this does not necessarily imply causation; however, a previous study did find, that the majority of stage I lung cancers were incidental findings [Citation11] and the current audit found that 86.6% of stage IA lung cancer cases were not under suspicion for lung cancer. This corresponds with experience from a single-center study. Here two time periods were compared; in the period 2013–2015, clinicians’ access to CT thorax was restricted, while a more liberal access to CT thorax was implemented in the period 2016–2018 [Citation22]. The study found a marked increase in the fraction of stage IA from 13.8% to 28.3%, with a more liberal access to low dose CT thorax, even though the majority of CT exam referrals came from in-hospital departments.
However, the regional differences regarding the relation between number of CTs per 1000 inhabitants and the fraction of patients diagnosed with stage IA lung cancer highlight the fact that the number of CT examinations is not the only factor influencing the stage distribution of lung cancer. The necessary resources for careful CT interpretation and proper follow-up regimens in accordance with guidelines should be put in place to minimize the risk of missing an opportunity to diagnose a lung cancer at an early stage. The clinical audit of stage IV lung cancer patients provides valuable learning points in this respect. In total, 73% of the pulmonary nodules/infiltrates that most likely developed into stage IV lung cancer were described by the radiologist. As seen in , all of these nodules/infiltrates had a size that required CT-based follow-up, but this did not happen. The following reasons for the lack of follow-up were found in the clinical audit: The radiologist describes the infiltrate but does not recommend follow-up; the radiologist recommends follow-up but the referring department does not perform CT follow-up; the CT exam is sent for assessment at the regional lung cancer investigation unit, where follow-up is recommended, but the referring department does not perform follow-up; the referring department uses chest x-ray for follow-up of pneumonia/diffuse infiltrates. This highlights the necessity for firm follow-up algorithms of incidental findings in CT thorax at the radiology and respiratory medicine departments.
The remaining 27% of the pulmonary nodules/infiltrates were not mentioned by the radiologist. These were smaller than the described infiltrates/nodules, with a mean diameter of 9.8 mm (range 5–24 mm) and sizes are comparable to results in a previous study on missed nodules developing into lung cancer [Citation23]. However, with nodule-specific training, thoracic radiologists can improve their skills of detecting pulmonary nodules [Citation24].
If the clinical audit also included patients in Stages II and III, it could have revealed more nodules/infiltrates that developed into a later stage of lung cancer. Earlier detection of these lung cancers could have resulted in better treatment options, such as surgery instead of concurrent chemoradiotherapy. However, the current study focused on stage IV patients, for whom curative treatments options are rare, and thus representing the most radical and clear consequence of a missed opportunity. The actual number of cases of missed early-stage lung cancer among the patients diagnosed with stage IV lung cancer might in fact have been higher, as some cases of lung cancer, typically with subsolid morphology, are slow growing and may require more than 2 years to progress from stage IA to IV. Our choice of an audit period of two years before diagnosis was partly dictated by a legal limit of five years from diagnosis for quality assessment studies and partly because 2 years is the usual follow-up period for solid pulmonary nodules.
This study has some limitations. Correlations between the number of CT examinations and the fraction of stage IA lung cancer do not necessarily imply causation, even though it seems likely, given that: (1) these small tumors are practically undetectable without the use of CT and (2) the majority of stage IA lung cancers are incidental findings on CT scans performed for many reasons other than suspicion of lung cancer. The clinical audit of stage IV patients assessed if previously detected pulmonary nodules/infiltrates were likely to be the origin of the later stage IV lung cancer. This was a subjective retrospective assessment based on the same location of the pulmonary nodule/infiltrate and the primary tumor of the later diagnosed stage IV lung cancer.
In conclusion, this study finds a likely relationship between the number of clinical CT thorax examinations and the fraction of stage IA lung cancer. The learning points from the clinical audit highlight that follow-up algorithms of incidental findings should be used in accordance with guidelines and that it should be unequivocally how the CT follow-up of pulmonary infiltrates is managed.
Disclosure statement
The authors report there are no competing interests to declare.
Data availability statement
Data on CT examinations in Denmark and lung cancer stage distribution are publicly available. Clinical audit data will be made available upon request and after approval of the hospital directors.
References
- Schockel L, Jost G, Seidensticker P, et al. Developments in X-ray contrast media and the potential impact on computed tomography. Invest Radiol. 2020;55(9):592–597.
- Number of examinations with computer tomography (CT) in selected countries as of 2019 [Internet]. 2022. [cited 28.06.22]. Available from: https://www.statista.com/statistics/283085/computer-tomography-examinations-in-selected-countries/
- Computed tomography exams [Internet]. 2021. [cited August 2022]. Available from: https://www.oecd-ilibrary.org/social-issues-migration-health/computed-tomography-ct-exams/indicator/english_3c994537-en
- Registries NAotNC. Cancer statistics. 2022. Available from: https://www-dep.iarc.fr/nordcan/dk/frame.asp
- Lumbreras B, Donat L, Hernandez-Aguado I. Incidental findings in imaging diagnostic tests: a systematic review. Br J Radiol. 2010;83(988):276–289.
- Kocher F, Lunger F, Seeber A, et al. Incidental diagnosis of asymptomatic non-small-cell lung cancer: a registry-based analysis. Clin Lung Cancer. 2016;17(1):62–67 e1.
- Orrason AW, Sigurdsson MI, Baldvinsson K, et al. Incidental detection by computed tomography is an independent prognostic factor for survival in patients operated for nonsmall cell lung carcinoma. ERJ Open Res. 2017;3(2):00106–2016.
- Quadrelli S, Lyons G, Colt H, et al. Clinical characteristics and prognosis of incidentally detected lung cancers. Int J Surg Oncol. 2015;2015:1–6.
- Raz DJ, Glidden DV, Odisho AY, et al. Clinical characteristics and survival of patients with surgically resected, incidentally detected lung cancer. J Thorac Oncol. 2007;2(2):125–130.
- Rampinelli C, Calloni SF, Minotti M, et al. Spectrum of early lung cancer presentation in low-dose screening CT: a pictorial review. Insights Imaging. 2016;7(3):449–459.
- Bredtoft EN, Madsen HH, Rasmussen TR. Stage I lung cancer patients with or without symptoms - are the patients different and should we treat them differently? Acta Oncol. 2021;60(9):1169–1174.
- Webpage. Dansk Lunge Cancer Register. 2022. Available from: www.lungecancer.dk
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39–51.
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381.
- MacMahon H, Naidich DP, Goo JM, et al. Guidelines for management of incidental pulmonary nodules detected on CT images: from the Fleischner Society 2017. Radiology. 2017;284(1):228–243.
- Slatore CG, Au DH, Gould MK. An official American thoracic society systematic review: insurance status and disparities in lung cancer practices and outcomes. Am J Respir Crit Care Med. 2010;182(9):1195–1205.
- Nations JA, Brown DW, Shao S, et al. Comparative trends in the distribution of lung cancer stage at diagnosis in the Department of Defense Cancer Registry and the surveillance, epidemiology, and end results data, 1989–2012. Mil Med. 2020;185(11–12):e2044–e2048.
- Henriksen DP, Rasmussen L, Hansen MR, et al. Comparison of the five Danish regions regarding demographic characteristics, healthcare utilization, and medication use–A descriptive cross-sectional study. PLOS One. 2015;10(10):e0140197.
- de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382(6):503–513.
- Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409.
- Becker N, Motsch E, Trotter A, et al. Lung cancer mortality reduction by LDCT screening-results from the randomized German LUSI trial. Int J Cancer. 2020;146(6):1503–1513.
- Hyldgaard C, Trolle C, Harders SMW, et al. Increased use of diagnostic CT imaging increases the detection of stage IA lung cancer: pathways and patient characteristics. BMC Cancer. 2022;22(1):464.
- Li F, Sone S, Abe H, et al. Lung cancers missed at low-dose helical CT screening in a general population: comparison of clinical, histopathologic, and imaging findings. Radiology. 2002;225(3):673–683.
- Digumarthy SR, Lo Gullo R, Levesque MH, et al. Cause determination of missed lung nodules and impact of reader training and education: simulation study with nodule insertion software. J Can Res Ther. 2020;16(4):780–787.