Abstract
Background
Transmembrane protein 27 (TMEM27/collectrin), a glycoprotein and homolog of angiotensin-converting enzyme 2 (ACE2), is a regulator of renal amino acid uptake in the proximal tubule and may have a protective role in hypertension. Two previous reports have shown that the absence of TMEM27 expression in clear cell renal cell carcinoma (ccRCC) correlates with poorer cancer-related survival. We report our findings of TMEM27 expression in ccRCC and clinical outcomes in an independent third cohort.
Material and Methods
We conducted a retrospective analysis to identify all 321 cases of ccRCC diagnosed between 2010 and 2015 at the University of Rochester Medical Center. The intensity of TMEM27 immunostaining on tumor tissue was semi-quantitatively graded on a scale of 0, 0.5, 1, 1.5, 2, 2.5, and 3 by a single pathologist, and correlated with tumor characteristics and survival.
Results
There was evidence of metastasis at time of nephrectomy in 36 (11.2%) cases, and at the latest follow-up in 70 (21.8%) cases. As of Spring 2021, 82 (25.5%) had died. TMEM27 staining intensity correlated inversely with various tumor characteristics. Kaplan-Meier survival analysis showed worse overall all-cause mortality (p = 0.02) and disease-free survival (p = 0.028) for tumors without any TMEM27 staining (0) compared to 0.5 or higher by log-rank test.
Conclusion
The absence of TMEM27 expression is associated with more aggressive tumor characteristics and poorer all-cause mortality and disease-free survival in ccRCC. TMEM27 may be a useful biomarker to assess cancer prognosis. Further studies are needed to better assess if TMEM27 is protective in RCC, and its potential role in active surveillance and prediction of response to target therapy.
Background
Collectrin is a 222-amino acid transmembrane glycoprotein (Transmembrane protein 27 (TMEM27)) with 47.8% sequence homology to angiotensin-converting enzyme-related carboxypeptidase (ACE2) [Citation1]. In addition to expression in the proximal tubule and collecting duct of the kidneys, collectrin expression has also been found in pancreatic β-cells, liver, lungs, intestinal epithelial cells, retina, brain, and vascular endothelial [Citation2] cells with various tissue-specific roles. In the kidneys, deletion of collectrin expression results in significant aminoaciduria, highlighting collectrin’s importance in renal amino acid transport [Citation3]. More recently, collectrin-deficient mice have been shown to develop hypertension with increased salt sensitivity [Citation2,Citation4], illustrating the protective role of intra-renal collectrin. Further, collectrin-deficient mice exhibit endothelial dysfunction with uncoupling of endothelial nitric oxide synthase and increased superoxide production, most likely due to decreased cellular uptake of L-arginine substrate [Citation4].
In 2020, there were over 430,000 new cases of kidney cancer and over 170,000 deaths due to kidney cancer with the highest incidence and mortality rates in Europe and North America [Citation5]. Renal cell carcinoma (RCC) accounts for approximately 90% of kidney cancers, with the most common histologic type being clear cell renal cell carcinoma (ccRCC) (75%) [Citation6]. Cigarette smoking, obesity, and hypertension have been found to be well-established risk factors for developing RCC [Citation7].
Two previous reports have shown that the absence of TMEM27 expression in clear cell renal cell carcinoma (ccRCC) correlates with poorer cancer-related survival [Citation8,Citation9]. Javorhazy et al. presented the first cohort of 486 patients with renal cell carcinoma and showed that TMEM27 expression inversely correlated with patient survival, tumor size, grade, stage, and metastasis at the time of operation [Citation9]. In fact, in patients undergoing nephrectomy for localized RCC, those without TMEM27 tumor expression had 3-fold higher cancer-specific mortality compared to patients with TMEM27 tumor expression. By immunohistochemistry (IHC) staining, TMEM27 appeared to be expressed on the cell membrane of tumor cells [Citation9]. In multivariate analysis of patients with localized disease, only TMEM27 expression and tumor stage were significant predictors of cancer-specific survival. Uhlen et al. provided the second cohort of 877 patients [Citation8] which was then analyzed by the Human Protein Atlas showing lower expression of TMEM27 was associated with worse survival (see link https://www.proteinatlas.org/ENSG00000147003-CLTRN/pathology/renal+cancer). Herein, we report our single-center findings of TMEM27 expression with clinicopathologic variables and outcomes in patients with ccRCC undergoing nephrectomy.
Material and methods
We conducted a retrospective analysis to identify all cases of ccRCC diagnosed between 2010 and 2015 at the University of Rochester Medical Center. Data collected included age, gender, and the variables used for pathologic staging using the College of American Pathologist (CAP) protocol: tumor size, tumor stage (using TNM staging), and Furhman grade. Additional characteristics including presence of sarcomatoid/rhabdoid features, presence of large vessel venous or lymphatic invasion, metastasis at time of nephrectomy and at follow up, and patient death (through March 31, 2021) were also obtained. A composite score consisting of a summation of the raw values [‘Tumor size,’ ‘TNM stage (pTx),’ ‘Furhman grade,’ ‘regional lymph nodes (pN),’ ‘sarcomatoid change or rhabdoid change present? (yes = 1, no = 0)’] was calculated. Further, a composite score with patient death [a summation of the composite score and the death event (+1)] was also calculated. Since the CAP protocol was used for pathologic staging based on size and extension of the tumor, data on tumor necrosis was not available, and therefore a modified SSIGN (stage, size, grade and necrosis) score was calculated without the tumor necrosis variable.
Immunohistochemistry staining
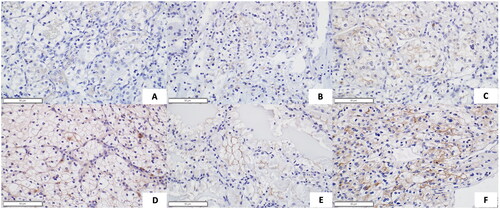
Tissue slides from partial or total nephrectomy specimens with ccRCC were stained for TMEM27. Immunostaining of TMEM27 was performed using paraffin embedded kidney tissues, following the instruction of the ImmPress kit (MP-7401, Vector Laboratories) with small modifications. Briefly, 4 µm sections were de-paraffinized and rehydrated through decreasing ethanol solutions in water. Antigen retrieval was performed using sodium citrate buffer (pH 6.0) for 10 min in boiling water. Rabbit anti-mouse polyclonal collectrin antibody (1:1000) (custom made for T. H. Le’s lab by Covance Immuno Technologies, Denver, PA, by generation against the synthetic peptide NDAFMTEDERLTPL as previously reported [Citation4]) was added to the slides for overnight incubation after blocking procedure. Hematoxylin was used for counterstaining. A single pathologist (H. Y. G. Choung) then analyzed the prepared tumor tissue slides and semi-quantitatively graded the intensity of TMEM27 immunostaining, using a scale of 0, 0.5, 1, 1.5, 2, 2.5, and 3 ().
Statistical analysis
A Pearson correlation coefficient and p-value for testing non-correlation was generated for TMEM27 staining intensity (0, 0.5, 1, 1.5, 2, 2.5 and 3) versus the following variables: tumor size, TNM stage (pT and pN), Furhman grade, sarcomatoid/rhabdoid changes present, large vessel venous invasion, lymphatic invasion, metastasis at time of nephrectomy, metastasis at follow-up, composite, ‘composite with death,’ and modified SSIGN score.
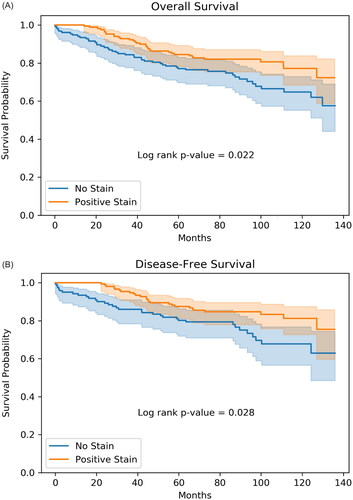
When variables were non-numeric, but represented an increasing progression, such as the TNM stage, they were transformed to increasing numerical values: 1a->1.1, 1 b->1.2, 2a->2.1, 2 b->2.2, 3a->3.1, 3 b->3.2, 3c->3.3. Analysis was performed using Python and the scipy toolkit. The Kaplan-Meier survival plots were generated using the lifelines survival analysis library. The first plot included all 321 subjects for overall survival (), and the second plot excluded patients with metastasis at time of nephrectomy (N = 285) for disease-free survival ().
Figure 2. (A) Kaplan–Meier analysis demonstrating the association of TMEM27 IHC staining intensity with overall survival of 321 patients from time of nephrectomy. No stain is tumor that has 0 stain for TMEM27. Positive stain includes tumors with staining intensity from 0.5 to 3.0. The shaded areas around the lines represent the 95% confidence band. (B) Kaplan–Meier analysis demonstrating the association of TMEM27 IHC with disease-free survival in patients without metastasis at time of nephrectomy (N = 285). No stain is tumor that has 0 stain for TMEM27. Positive stain includes tumors with staining intensity from 0.5 to 3.0. The shaded areas around the lines represent the 95% confidence band.
Results
There was a total of 321 cases of ccRCC diagnosed between 2010 and 2015. Thirty-six cases (11.2%) showed evidence of metastasis at the time of nephrectomy. 70 cases (21.8%) showed evidence of metastasis at the latest follow-up. There were 82 deaths (25.5%) as of Spring 2021 (). Of the 321 cases, 60% were males. There was no significant difference found in the proportions of sexes between the 0 staining and those with 0.5 − 3.0 staining groups, using a simple normal test for proportions (python statsmodels). The two staining groups were also of similar age.
Table 1. Patient demographics based on intensity of TMEM27 immunohistochemistry staining.
Similar to findings by Javorhazy et al. IHC staining showed that TMEM27 is expressed primarily on the cell membrane of tumor cells. TMEM27 staining intensity correlated inversely with a variety of tumor characteristics including tumor size, primary tumor (pT) stage, Fuhrman grade, large vessel venous invasion, and metastasis at time of nephrectomy (). The intensity of TMEM27 immunostaining also inversely correlated with the composite clinical characteristic score both with/without including patient death in the score. Similarly, the intensity of TMEM27 immunostaining also inversely correlated with the SSIGN score.
Table 2. Correlation between TMEM27 staining and clinical characteristics.
Kaplan-Meier analysis of the entire cohort of 321 patients showed worse overall all-cause mortality for tumors without any TMEM27 staining (No stain) compared to staining intensity of 0.5 or higher (Positive Stain), p = 0.02 by log-rank test (). Kaplan–Meier analysis of patients with localized disease at the time of nephrectomy (N = 285) also showed worse disease-free survival for those with tumors having no TMEM27 staining (No stain) compared to those with staining intensity 0.5 or higher (Positive Stain), p = 0.028 by log-rank test ().
Discussion/conclusion
We show here that the absence of TMEM27 expression is associated with more aggressive tumor characteristics and poorer overall all-cause mortality and disease-free survival in ccRCC. We found that the lack of TMEM27 expression correlated inversely with other known prognostic clinicopathologic variables including tumor size, stage, and grade [Citation10]. However, while Javorhazy et al. found that lack of TMEM27 expression also correlated with the presence of sarcomatoid dedifferentiation, which is known to have an aggressive clinical phenotype with poor outcomes [Citation11,Citation12], we did not find such correlation.
Similar to Javorhazy et al., we found that lack of TMEM27 expression was associated with metastasis at time of nephrectomy, though it did not seem to correlate with metastasis at the time of follow up. Approximately 90% of metastasis at time of nephrectomy occurred in the lung, with a few in both the lung and spine, and rare cases involving only liver or small bowel as non-lung sites of metastasis, precluding analysis of metastatic site correlation with TMEM27. We also found that lack of TMEM27 expression was associated with a composite score of all the tumor characteristics analyzed, both with and without incorporating patient death, as well as a modified SSIGN score.
Strengths of our study include a relatively large sample size of patients and the use of a single pathologist to semi-quantitively grade the intensity of TMEM27 staining, thereby limiting the potential for observer bias. Weaknesses of our study include that the cases analyzed were from a single center thereby raising uncertainty as to whether our findings can be generalized to a more diverse population. Nevertheless, our findings are congruent with that of two other independent studies published over 4 years ago and further suggest that lack of TMEM27 expression may play a predictive role in estimating prognosis and possibly even guiding treatment plans. Our data was gathered from patients diagnosed from 2010 to 2015, a time period during which virtually all patients with metastatic disease were treated with targeted therapies. Our small number of cases of metastasis at diagnosis is insufficient to assess response to target therapy.
The mechanism by which loss of TMEM27 is associated with more aggressive tumor characteristics is unknown. RCC is now generally considered a metabolic disease. Mutations in regulatory genes involved in aerobic glycolysis, fatty acid metabolism, and tryptophan, glutamine, and arginine utilization have been discovered [Citation13,Citation14]. As TMEM27 is an essential chaperone of amino acid transporters, it is tempting to speculate that loss of TMEM27 alters metabolic characteristics in tumor cells. In this regard, mice lacking TMEM27 display increased insulin sensitivity, and up-regulation of energy-generating processes, including carbohydrate and lipid oxidation [Citation15]. As cancer progresses, its metabolic properties evolve [Citation16,Citation17]. Metabolic alteration has been observed to correlate with worse prognosis in other urothelial cancers. For example, in primary T1HG/G3 non-muscle invasive bladder cancer treated with transurethral resection of the bladder (TURB), patients with type 2 diabetes mellitus (T2DM) had significantly worse five-year recurrence-free survival compared to those without T2DM [Citation18]. Metabolic profiling of bladder cancer has identified biochemical pathways including glycolysis, the tricarboxylic acid (TCA) cycle, fatty acid β-oxidation, and amino acid metabolism. In prostate cancer, increased body mass index correlates with aggressive disease, and metabolic profiling has shown that there is accumulation of metabolic intermediates and increased expression of genes in the TCA cycle, as well as induction of de novo lipogenesis and cholesterogenesis [Citation19]. It should be noted, however, that TMEM27 has not been shown to be prognostic in urothelial cancer (see link https://www.proteinatlas.org/ENSG00000147003-CLTRN/pathology/urothelial+cancer) nor in prostate cancer (see link https://www.proteinatlas.org/ENSG00000147003-CLTRN/pathology).
Further studies are needed to better determine if TMEM27 has a role in RCC metabolism, as well as a clinical prognostic factor in RCC. Moreover, future direction would be to explore the prognostic utility of TMEM27 expression in predicting indolent versus aggressive phenotypes of small renal masses on biopsy specimens to refine the selection of appropriate active surveillance candidates, particularly in those who may not be good surgical candidates, and to predict response to targeted therapy.
Ethical approval
This research was performed under approval of an institutional review board (STUDY00005344) and all ethical principles and guidelines for the protection of human subjects were followed.
Author contributions
THL conceived of and initiated the study. RG and LLR performed the chart review and data collection. HYGC assessed immunostaining and performed the pathology portion of the data collection. DXG performed statistical analysis. RG and THL wrote the first draft of the manuscript. RG, PMR, and THL critically revised the work. TB and LC assisted in slide collection and performed the immunostaining of all case slides. All authors read and approved the final manuscript.
Acknowledgement
The authors are grateful to Sierra Kovar for her excellent technical support and guidance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, [RG], upon reasonable request.
References
- Zhang H, Wada J, Hida K, et al. Collectrin, a collecting duct-specific transmembrane glycoprotein, is a novel homolog of ACE2 and is developmentally regulated in embryonic kidneys. J Biol Chem. 2001;276(20):17132–17139.
- Chu PL, Gigliotti JC, Cechova S, et al. Renal collectrin protects against Salt-Sensitive hypertension and is downregulated by angiotensin II. J Am Soc Nephrol. 2017;28(6):1826–1837.
- Malakauskas SM, Quan H, Fields TA, et al. Aminoaciduria and altered renal expression of luminal amino acid transporters in mice lacking novel gene collectrin. Am J Physiol Renal Physiol. 2007;292(2):F533–44.
- Cechova S, Zeng Q, Billaud M, et al. Loss of collectrin, an angiotensin-converting enzyme 2 homolog, uncouples endothelial nitric oxide synthase and causes hypertension and vascular dysfunction. Circulation. 2013;128(16):1770–1780.
- Kidney Fact Sheet. International Agency for Research on Cancer: the Global Cancer Observatory; 2020.
- Znaor A, Lortet-Tieulent J, Laversanne M, et al. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol. 2015;67(3):519–530.
- Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol. 2010;7(5):245–257.
- Uhlen M, Zhang C, Lee S, et al. A pathology atlas of the human cancer transcriptome. Science. 2017;357(6352):eaan2507.
- Javorhazy A, Farkas N, Beothe T, et al. Lack of TMEM27 expression is associated with postoperative progression of clinically localized conventional renal cell carcinoma. J Cancer Res Clin Oncol. 2016;142(9):1947–1953.
- Rioux-Leclercq N, Karakiewicz PI, Trinh QD, et al. Prognostic ability of simplified nuclear grading of renal cell carcinoma. Cancer. 2007;109(5):868–874.
- Delahunt B, Cheville JC, Martignoni G, Members of the ISUP Renal Tumor Panel, et al. The international society of urological pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters. Am J Surg Pathol. 2013;37(10):1490–1504.
- Bakouny Z, Braun DA, Shukla SA, et al. Integrative molecular characterization of sarcomatoid and rhabdoid renal cell carcinoma. Nat Commun. 2021;12(1):808.
- Lucarelli G, Loizzo D, Franzin R, et al. Metabolomic insights into pathophysiological mechanisms and biomarker discovery in clear cell renal cell carcinoma. Expert Rev Mol Diagn. 2019; 19(5):397–407.
- Qi X, Li Q, Che X, et al. The uniqueness of clear cell renal cell carcinoma: summary of the process and abnormality of glucose metabolism and lipid metabolism in ccRCC [review. Front Oncol. 2021;11:727778.
- Malakauskas SM, Kourany WM, Zhang XY, et al. Increased insulin sensitivity in mice lacking collectrin, a downstream target of HNF-1α. Mol Endocrinol. 2009;23(6):881–892.
- Faubert B, Solmonson A, DeBerardinis RJ. Metabolic reprogramming and cancer progression. Science. 2020;368(6487):eaaw5473.
- di Meo NA, Loizzo D, Pandolfo SD, et al. Metabolomic approaches for detection and identification of biomarkers and altered pathways in bladder cancer. Int J Mol Sci. 2022;23(8):4173.
- Ferro M, Katalin MO, Buonerba C, et al. Type 2 diabetes mellitus predicts worse outcomes in patients with high-grade T1 bladder cancer receiving bacillus Calmette-Guerin after transurethral resection of the bladder tumor. Urol Oncol. 2020;38(5):459–464.
- Lucarelli G, Loizzo D, Ferro M, et al. Metabolomic profiling for the identification of novel diagnostic markers and therapeutic targets in prostate cancer: an update. Expert Rev Mol Diagn. 2019;19(5):377–387.