Background
The systemic treatment of patients with metastatic renal cell carcinoma (mRCC) has evolved tremendously during the last couple of decades and survival has been significantly improved [Citation1]. To further improve treatment outcome, current research focuses on the efficacy of combination treatment or new drugs, but also in risk of introducing more, new or unknown side effects [Citation2–7]. In addition to the medical treatment, other interventions are explored to improve overall patient outcome. One intervention is the collection and use of patient-reported outcome (PRO). The U.S. Food and Drug Administration (FDA) defines PRO as ‘any report of the status of a patient’s health condition that comes directly from the patient without interpretation of the patient’s response by a clinician or anyone else’ [Citation8]. Basch et al. tested the active use of weekly electronic PRO (ePRO) monitoring of patients undergoing treatment for metastatic cancer in a randomized phase III study [Citation9,Citation10]. Their results showed significantly better quality of life and survival in the intervention arm compared to standard care. In the study, PRO data were assessed and actioned upon in real-time (an active use of PRO), thereby benefitting the patient who reported data. An active use of PRO enables individualized patient care, strengthens clinical decision making, and has the potential to detect side effects and symptoms that would otherwise be overlooked or reported with delay. This article describes the PRORECECA, an acronym for Patient-Reported Outcomes by patients with metastatic REnal CEll CArcinoma, study as presented in the protocol version 1 dated 29 September 2022. In the PRORECECA study, we focus on the effect of an active use of ePRO and physical function. Physical function can be defined as ‘physical abilities that are considered essential for maintaining independence’ [Citation11] and is an important part of quality of life assessment. In the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C-30 (EORTC QLQ-C30), five questions (physical domain) aim at measuring different aspects of the patient’s physical function with a score from 1 to 4 indicating the degree of impaired physical function.
In the PRORECECA study, we will test the following: Can weekly active monitoring of patient-reported symptoms after three months improve self-reported physical function in patients with mRCC receiving systemic therapy?
The hypothesis is that ePRO will improve the rate of patients who after three months experience a 10-point improvement in self-reported physical function, as measured with the Physical Function domain of EORTC QLQ-C30, from 10% in the standard arm to 30% in the intervention arm.
Material and methods
Patients
Patients with mRCC initiating first or second line standard therapy with either immunotherapy (IO), tyrosine kinase inhibitors (TKI), or IO in combination with TKI can participate in the study. Additional inclusion criteria are age ≥ 18 years, performance status ≤ 2, ability to read Danish, no serious cognitive impairment, and that the patient has given written informed consent.
Exclusion criteria are no smartphone, participation in other interventional studies, earlier participation in PRORECECA e.g., when changing from first to second line of treatment, deprivation of liberty or guardianship, and dementia, mental alteration, or psychiatric disease that can compromise informed consent from the patient and/or adherence to the protocol and the monitoring of the trial.
If the patient is a candidate for participation in the PRORECECA study, a clinician will inform the patient about the study and obtain informed content if the patient wishes to participate. The patients can be recruited when starting first or second line systemic therapy for mRCC.
Design
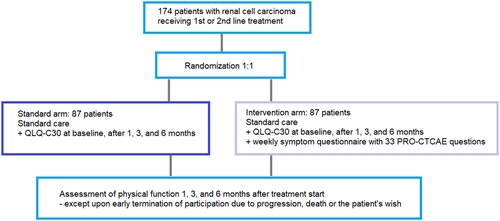
The PRORECECA study, clinicaltrials.gov identifier: NCT05135832, is an investigator-initiated, national, Danish multicenter randomized two-armed controlled trial connected to the Danish Renal Cancer Group. The study design can be seen in and the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) 2013 Checklist and SPIRIT-PRO Protocol Guidance Checklist can be seen in Supplementary Materials 1 and 2 [Citation12]. After signing informed consent, patients are randomized into the arms in the ratio 1:1. The randomization will be carried out automatically when a clinician is creating the patient’s profile in Research Electronic Data Capture (REDCap), where study data relating to the patient is entered. Randomization will be conducted in a block size of four and stratified into groups according to risk group, treatment line, treatment type, and performance status.
Experimental arm
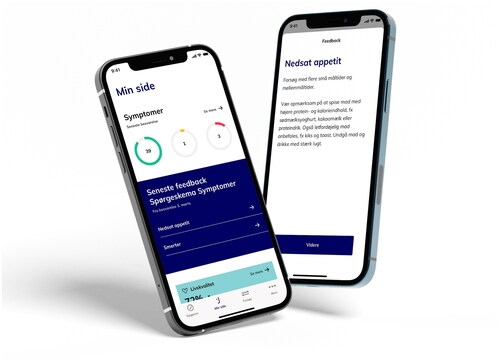
Patients in the experimental arm will receive an electronic PRO-Common Terminology Criteria for Adverse Events (ePRO-CTCAE) questionnaire with selected questions in an app every week. An example of the app can be seen in . If the weekly reported symptoms exceed a predefined threshold of severity, the patient will get predefined feedback to adjust supportive care or is advised to contact the hospital. The reported symptoms are sent to the hospital where clinicians review the reports every weekday and can contact the patient to initiate or improve supportive care. The ePRO questionnaires are added to standard treatment and symptoms revealed will be treated according to standard procedures. The selected ePRO-CTCAE questions, alert algorithm and threshold for alerts can be seen in Supplementary Material 3.
The patients will also receive EORTC QLQ-C30 including the Physical Function domain in the app at baseline and after 1, 3, and 6 months of participation. The questionnaire can be seen in Supplementary Material. 4
At the end of treatment, the patient will answer a validated Patient-Reported Experience Measure (PREM) questionnaire for evaluating the patient satisfaction with the questionnaires [Citation13] (Supplementary Material 5).
Control arm
The patients in the control arm will also receive EORTC QOL-C30 in the Danish secure digital platform ‘e-Boks’ used for communication between the public sector and citizens of Denmark. The questionnaires are sent to the patients at baseline and after one, three, and six months of participation in the study.
Study flow
First patient is expected to be enrolled in winter 2022 and inclusion is expected to take a year and a half. Patients will be followed up to six months after signing informed consent. Further follow-up is not planned. The patients’ participation in the study may end before the six months if the patients want to discontinue, the patients change systemic treatment or due to death.
Electronic patient-reported outcome
The weekly ePRO-CTCAE questionnaire consists of 32 questions regarding symptoms and side effects from the PRO-CTCAE item Library. A group of medical oncologists with expertise in treating mRCC or ePRO selected the questions based on the expected frequencies of symptoms and side effects. Also, rare but severe symptoms are part of the questionnaire. The questionnaires are the same regardless of the patient’s oncological treatment.
In the intervention arm, the patients receive the questionnaires in an app. When the patients answer the questionnaires, they will get immediate feedback in the form of advices regarding the symptoms in the app. The patient can continuously find the advices in the app. The advices are predefined and based on an algorithm linked to the severity of the symptoms or side effects that the patient reports. The algorithm was developed by the expert group and the threshold for intervention, and type of intervention was determined individually for each symptom.
The clinician can access both the answers of the symptom questionnaires and quality of life-questionnaires, but the latter is a passive collection of ePRO. Clinicians are encouraged to use the ePRO reports both between hospital visits and during consultation as a starting point for dialogue between the clinician and patient.
The app is developed by the app provider Journl who is specialized in ePRO apps. Journl is ISO 13485 and ISO 27001 certified. The app and the questionnaires are in Danish. The patient will download the app, create a user profile, and answer the first questionnaires in relation to the first treatment. The patient will receive daily notifications from the app if there is an unanswered questionnaire.
Endpoints
Primary endpoint is self-reported physical function after three months. Secondary endpoints are health-related quality of life, number and length of admissions, symptom management and number of contacts to the oncology departments, number and length of treatment pause or treatment delay, and patient satisfaction regarding the use of ePRO.
In addition to compare the patients in the two arms, analyses of the endpoints in the following groups will be performed:
Patients receiving different treatment options (TKI, IO, or TKI and IO)
First versus second line treatment
Risk group (poor versus intermediate versus good)
Performance Status 0–1 versus 2
Sample size
A total of 174 patients will be included in the study with 87 patients in each arm. The sample size is based on data from Checkmate 214 [Citation4,Citation14]. With 72 patients in each arm, we can reject the null hypothesis that the failure rates for experimental and control subjects are equal with a probability (power) 0,8. When stratifying in the above mentioned groups (see ‘Endpoints’) a heterogenicity calculation is performed. This requires a 20% larger sample size and the number of patients in each arm is increased with 15 (≈ 20%). The Type I error probability associated with this test of this null hypothesis is 0,05. We will use a continuity-corrected chi-squared statistic or Fisher’s exact test to evaluate this null hypothesis.
Data collection and statistics
Baseline information on age, time of diagnosis, gender, histology (extracted from the pathology database), TNM stage, treatment type, treatment line, Eastern Cooperative Oncology Group performance status, and risk group will be noted in REDCap. A baseline quality of life questionnaire will be completed. During the study period, we will collect information regarding health-related quality of life, number and length of hospital admissions, symptom management and number of contacts to the departments (both phone and attendance). If or when treatment is discontinued the duration of the treatment and reasons for discontinuation is registered.
The analyses of ePRO data will include descriptive statistics for both changes from baseline and observed scores after three and six months of participation in the study.
An interim analysis on the secondary endpoint ‘symptom management’ will be performed 3 months after inclusion of patient number 80. The focus in the analysis is to examine whether the selected ePRO-questions are relevant for the patient group.
Ethics declaration
The PRORECECA study will be reviewed by the Medical Research Ethics Committee, will follow General Data Protection Regulation, and is registered at the Capital Region of Denmark.
The results of the study will be reported both in a scientific journal, on clinicaltrials.gov and in a letter to the patients who, in connection to enrollment or during the study, have expressed interest in the results.
Conclusion
Results from the ongoing QUANARIE trial focuses on the patients’ compliance with routine electronic monitoring of health-related quality of life in patients with mRCC are presently awaited [Citation15]. Our PRORECECA study will further contribute with knowledge about the effect of active use of ePRO in patients with mRCC receiving oncological treatment on self-reported physical function. We hypothesize that addition of active ePRO to standard of care can improve patient outcome measured as self-reported physical function.
| Abbreviations | ||
| EORTC QLQ-C30 | = | The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C-30; |
| ePRO | = | Electronic patient-reported outcome; |
| ePRO-CTCAE | = | Electronic PRO-Common Terminology Criteria for Adverse Events; |
| FDA | = | The U.S. Food and Drug Administration; |
| IO | = | Immunotherapy; |
| mRCC | = | Metastatic renal cell carcinoma; |
| PREM | = | Patient-reported experience measure; |
| PRO | = | patient-reported outcome; |
| REDCap | = | Research Electronic Data Capture; |
| SPIRIT | = | Standard Protocol Items: Recommendations for International Trials; |
| TKI | = | Tyrosine kinase inhibitors |
Supplemental Material
Download PDF (187.5 KB)Supplemental Material
Download PDF (60.4 KB)Supplemental Material
Download PDF (162.2 KB)Supplemental Material
Download PDF (888.3 KB)Supplemental Material
Download PDF (116.8 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Due to the nature of the research and the strict Danish interpretation of the GDPR legislation, supporting data will not be available.
Additional information
Funding
References
- McKay RR, Bosse D, Choueiri TK. Evolving systemic treatment landscape for patients with advanced renal cell carcinoma. J Clin Oncol. 2018;36(36):3615–3623.
- Powles T, Rini B. Novel agents and drug development needs in advanced clear cell renal cancer. J Clin Oncol. 2018;36(36):3639–3644.
- Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103–1115.
- Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–1290.
- Motzer R, Alekseev B, Rha SY, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384(14):1289–1300.
- Choueiri TK, Powles T, Burotto M, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829–841.
- Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116–1127.
- Administration FAD. Guidance for industry on patient-reported outcome measures: use in medical product development to support labeling claims; availability; 2009. Available from: https://www.federalregister.gov/documents/2009/12/09/E9-29273/guidance-for-industry-on-patient-reported-outcome-measures-use-in-medical-product-development-to
- Basch E, Deal AM, Dueck AC, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318(2):197–198.
- Basch E, Deal AM, Kris MG, et al. Symptom monitoring with Patient-Reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557–565.
- Atkinson TM, Stover AM, Storfer DF, et al. Patient-reported physical function measures in cancer clinical trials. Epidemiol Rev. 2017;39(1):59–70.
- Calvert M, Kyte D, Mercieca-Bebber R, et al. Guidelines for inclusion of patient-reported outcomes in clinical trial protocols: the SPIRIT-PRO extension. JAMA. 2018;319(5):483–494.
- Tolstrup LK, Pappot H, Zangger G, et al. Danish translation, cultural adaption and initial psychometric evaluation of the patient feedback form. Health Qual Life Outcomes. 2018;16(1):77.
- Cella D, Grunwald V, Escudier B, et al. Patient-reported outcomes of patients with advanced renal cell carcinoma treated with nivolumab plus ipilimumab versus sunitinib (CheckMate 214): a randomised, phase 3 trial. Lancet Oncol. 2019;20(2):297–310.
- Mouillet G, Fritzsch J, Paget-Bailly S, et al. Health-related quality of life assessment for patients with advanced or metastatic renal cell carcinoma treated with a tyrosine kinase inhibitor using electronic patient-reported outcomes in daily clinical practice (QUANARIE trial): study protocol. Health Qual Life Outcomes. 2019;17(1):25.