Abstract
Background
The aim of this study was to assess the association between radiological and histopathological response after neoadjuvant radiotherapy (nRT) in soft tissue sarcoma (STS), as well as the prognostic value of the different response evaluation methods on the oncological outcome.
Methods
A retrospective cohort of patients with localized STS of the extremity and trunk wall, treated with nRT followed by resection were included. The radiological response was assessed by RECIST 1.1 (RECIST) and MR-adapted Choi (Choi), histopathologic response was evaluated according to the EORTC-STBSG recommendations. Oncological outcome parameters of interest were local recurrence-free survival (LRFS), disease metastases-free survival (DMFS), and overall survival (OS).
Results
For 107 patients, complete pre- and postoperative pathology and imaging datasets were available. Most tumors were high-grade (77%) and the most common histological subtypes were undifferentiated pleomorphic sarcoma/not otherwise specified (UPS/NOS, 40%), myxoid liposarcoma (MLS, 21%) and myxofibrosarcoma (MFS, 16%). When comparing RECIST to Choi, the response was differently categorized in 58%, with a higher response rate (CR + PR) with Choi. Radiological responders showed a significant lower median percentage of viable cells (RECIST p = .050, Choi p = .015) and necrosis (RECIST p < .001), and a higher median percentage of fibrosis (RECIST p = .005, Choi p = .008), compared to radiological non-responders (SD + PD). RECIST, Choi, fibrosis, and viable cells were not significantly associated with altered oncological outcome, more necrosis was associated with poorer OS (p = .038).
Conclusion
RECIST, Choi and the EORTC-STBSG response score show incongruent results in response evaluation. The radiological response was significantly correlated with a lower percentage of viable cells and necrosis, but a higher percentage of fibrosis. Apart from necrosis, radiological nor other histopathological parameters were associated with oncologic outcomes.
Background
Radiotherapy (RT) is an important component of multimodality treatment in localized extremity and trunk wall soft tissue sarcoma (STS). RT contributes to an improved local control after limb-sparing surgery [Citation1]. Neoadjuvant RT (nRT) has equivalent oncological outcomes compared to adjuvant RT but has several advantages such as the possibility to monitor the therapy response. However, the definition of response to RT is not well defined and can be determined by both radiological and histopathological assessment.
For radiological response, Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 based on the pre- and post-RT magnetic resonance imaging (MRI) is the most commonly used method to evaluate therapy response [Citation2]. However, several reports suggest that RECIST is a suboptimal method in STS since it only includes changes in tumor size. The Choi response criteria were developed to better evaluate Imatinib response in GIST and include changes in tumor density as measured by the attenuation coefficient Hounsfield unit [Citation3]. Nevertheless, imaging remains a noninvasive surrogate for the actual therapy-induced changes in tissue that can be observed through histopathological examination.
Histopathological evaluation represents another challenge in deciphering STS response to treatment, as different histopathological characteristics of pretreated and resected tumors have been described without unequivocal results. Given this lack of a standardized approach, the European Organization for Research and Treatment of Cancer – Soft tissue and Bone Sarcoma Group (EORTC-STBSG) has compiled recommendations for both histopathological and radiological examination and reporting [Citation4,Citation5]. However, these recommendations have not been widely validated [Citation6], nor do they provide cut-off scores for prognostic relevance.
To date no clear association has been found between radiological and histopathological response, and also the prognostic value of radiological or histopathological response characteristics for an oncological outcome is not well established. Therefore, the aim of this study was to further define the response evaluation after nRT in localized STS.
2. Methods
2.1. Patients and treatment
This retrospective single-center study was approved by the Institutional Review Board (no. IRBd19-306, The Netherlands Cancer Institute, Amsterdam, The Netherlands). Between January 2010 and July 2019, adult (≥18 years) patients were included with localized STS of trunk wall (twSTS) or extremities (eSTS), who had received nRT followed by definitive resection. Patients who had received other neoadjuvant therapies (nT, e.g., systemic therapy or isolated limb perfusion) or lacked (evaluable) contrast-enhanced MRI studies were excluded.
Histopathology
To be eligible, complete histopathological examination reports had to be available, both from the initial biopsy at diagnosis, as well as from the resection specimen. All biopsies and resection specimens were diagnosed and described by experienced sarcoma pathologists only (HB, PS, and EB). When the report did not describe the full set of parameters as needed for the study, specimens were reassessed by an experienced sarcoma pathologist. The pathologists were blinded to radiological outcomes. For the response evaluation, resection specimens were evaluated according to standard protocols; parameters of interest from the resection specimen included total percentage viable/stainable cells with corresponding final response score as described by the EORTC-STBSG [Citation5], the total percentage necrosis, and total percentage fibrosis/hyalinization (named ‘fibrosis’ from now on). Although Wardelmann et al. propose to refer to viable cells as ‘stainable cells’, we retain the term vital cells in this study [Citation5]. Pathological complete response (pCR) was defined as no viable tumor left in the resection specimen. The percentage of necrosis from the initial tumor biopsy was not reported in this study.
Imaging
MRI protocol
Only MRI scans with at least two orthogonal acquisition plains and the following available sequences were considered: T2-weighted-imaging (-WI), fat-suppressed T2-WI or proton density sequences, fat-suppressed T1-WI after Gadolinium–chelates injection, including subtraction imaging. Fat suppression techniques included short TI inversion recovery and Fat–Sat and Dixon methods. MRI’s were performed on both 1.5 T and 3.0 T MRI systems and contrast administration was performed in the portal/late phase as per good clinical practice. Baseline MRI performed in referral centers had to fulfill the aforementioned imaging standards to meet the inclusion criteria. MRI analysis was performed by two radiologists (NG and AB) with respectively 5 and 15 years of experience in musculoskeletal tumors, using a picture archiving and communication system workstation (Vue PACS, Carestream Health, Rochester, NY, USA). One radiologist (NG) revised the whole set of MRI scans; a second radiologist (AB) was consulted on a number of difficult cases. Both radiologists were blinded to histopathological outcomes.
RECIST and MR-adapted Choi
Tumor response was assessed according to both RECIST [Citation2] and MR-adapted Choi criteria (Choi). RECIST and Choi evaluations were obtained by measuring the tumor’s largest diameter and by quantitatively evaluating tumor enhancement, respectively. For RECIST evaluation, measures were performed on the most convenient MR sequence available, usually T2-WI. For Choi evaluation, measurements were performed according to the method previously described by Stacchiotti et al. [Citation7,Citation8], and the 3D Slicer 4.10.2 (www.slicer.org) was used for the segmentation of the tumor. The response was categorized as responders, consisting of complete response (CR) and partial response (PR), or non-responders, consisting of stable disease (SD) and progressive disease (PD) in both RECIST and Choi, see supplementary material. Tumor changes (both size and volume) were categorized as decreased (<95% of initial measurement), stable (±5% of initial measurement), or increased (>105% of initial measurement).
Study objectives
The primary goal of this study was to examine the association between histopathological features of the resected tissue with the radiological changes based on RECIST and Choi after nRT. The secondary goal was to examine the prognostic value of both histopathological and imaging characteristics, related to different oncological outcome parameters. Outcome parameters of interest were local recurrence-free survival (LRFS), distant metastases-free survival (DMFS), and overall survival (OS). LRFS was calculated as the time from surgery to local recurrence (LR) during follow-up (FU), as suspected on imaging or confirmed with a biopsy. Tumors categorized as LR were excluded from this analysis. DMFS was calculated from surgery (disease free) to distant metastasis (DM), patients with synchronous DM at diagnosis were excluded from this analysis. OS was calculated from the time since diagnosis to the last FU moment or death.
Statistical analysis
All analyses were performed in IBM SPSS 25.0 for Windows (version 25) with a significance level of α = 0.05. Median values are presented with ranges. Numerical and categorical variables with paired measurements were compared with the Wilcoxon signed rank test; binary variables with paired measurements were compared using the McNemar test. Kaplan–Meier curves were used to estimate survival curves, and survival differences (univariate) were calculated using the log-rank test. Patients with loss to follow-up were censored. Multivariate analyses were performed with Cox proportional hazard models to identify factors that influence the oncological outcome parameters as described above. Cohen’s κ was used to determine the probability-adjusted measure of agreement between the two imaging response criteria (RECIST and Choi).
Results
Patient, tumor, and treatment characteristics
We included a total of 107 localized STS patients who had received nRT followed by definitive surgery. Most common histological subtypes were undifferentiated pleomorphic sarcoma/not otherwise specified (UPS/NOS, 40%), myxoid liposarcoma (MLS, 21%), and myxofibrosarcoma (MFS, 16%). 77% were high-grade tumors. Of the patients in whom STS was diagnosed elsewhere after whoops resection (6%) received radiotherapy in between the whoops and the resection. For all patient, tumor, and treatment characteristics, see .
Table 1. Patient, tumor, and treatment characteristics (total n = 107).
Most patients received a dose of 50 Gy in 25 fractions (79%), second most applied regimen was 36 Gy applied in 18 fractions (9%) as part of a study on dose reduction of preoperative radiotherapy in myxoid liposarcomas [Citation9]. nRT was in all cases followed by resection of the (residual) STS lesion with an interval of 5–8 weeks to allow the acute radiation-associated toxicity to resolve.
Radiological response
Both axial maximum tumor diameter and tumor volume showed a non-significant change when comparing before and after nRT (respectively p = .150 and p = .597), see . The tumor diameter had decreased by 43% (n = 46) with a median change of −17% in axial diameter (IQR −31 to −8), which would be classified as SD according to RECIST criteria (see supplementary material). The tumor diameter had increased by 30% (n = 32) with a median change of +21% (IQR 12–34), which would be classified as PD according to RECIST. The tumor diameter was stable at 27% (n = 29). The tumor volume decreased in 45% (n = 48) with a median change of −46% (IQR −24 to −61), stable in 9% (n = 10), or had increased in 46% (n = 49) with a median change of +50% (IQR 31–100).
When performing subanalysis according to histological subtypes, we observed that MLS showed a significant decrease in axial diameter (p = <.001) and volume (p = <.001). A decrease in diameter and/or volume were both seen in 86% of MLS. The diameter decreased from a median of 7.50 cm (IQR 5.58−9.15) to 5.50 cm (IQR 4.08−7.08), the volume decreased from a median of 238.19 cm3 (IQR 48.10−632.31) to 97.71 cm3 (IQR 31.74−271.98). The median diameter decrease was −26% (IQR −18 to −34) and the median volume decrease was −56% (IQR −47 to −64). Other histological subtypes showed no significant changes.
The response was categorized differently according to Choi in 62 cases (58%) when compared to the RECIST response. Favorable response (CR + PR) was more often diagnosed according to Choi compared to RECIST, respectively 67% (n = 72) vs. 13% (n = 14), see . Cohen’s κ was 0.229 (p < .001), which reflects a fair agreement between the two response criteria. Two tumors showed a complete radiological response after nRT (1× leiomyosarcoma, 1× MFS). Differences between subtypes are depicted in .
Figure 1. Radiological response evaluation criteria; RECIST vs. Choi. Patients are color-coded based on the RECIST category; the second graph and table visualize the change in classification when classified according to Choi.
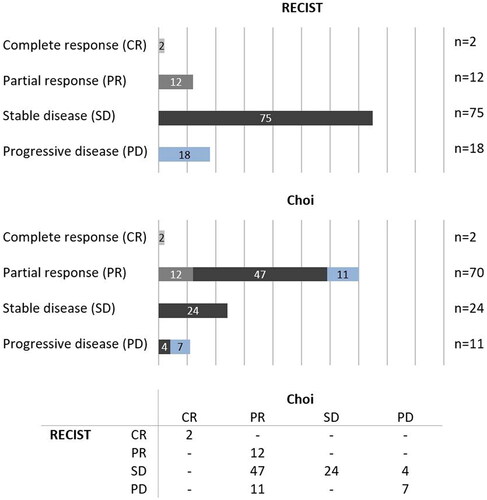
Table 2. Histopathological tumor characteristics after nRT and radiological response criteria per STS subtype.
Histopathological response
Resection margins were classified as R0 at 83% and as R1 at 17%. Eight patients (7%) had a pCR: 4× UPS/NOS, 3× MLS, and 1× MFS. A residual viable tumor was present in the remaining 93% and the overall median percentage of viable cells was 30% (IQR 10–65), with most responses classified as EORTC-STBSG response score E (≥50% viable tumor). Necrosis was present in 64% of the cases and the overall median necrosis was 10% (IQR 0–50). Fibrosis was present in 82% of the cases with an overall median of 25% fibrosis (IQR 5–60), see . Of note, the two tumors that showed a complete radiological response were recovered in the surgical specimen and showed the following histopathological response: 1.2 cm with 100% viable tumor and 2.5 cm with 60% viable tumor and 40% fibrosis. The histopathological characteristics varied between the different STS subtypes, see . Necrosis was significantly different between the different size categories (p = <.001). Post hoc analysis revealed a significant difference between ≤5 cm (median 0% necrosis) and 5–10 cm (median 20% necrosis, p = .001) and between ≤5 cm (median 0% necrosis) and >10 cm (median 35% necrosis).
Association of radiological and histopathological response evaluation
For the histopathological characteristics per the radiological response criteria method, see .
Table 3. Histopathological characteristics per radiological response criteria method.
Radiological responders showed a significant lower median percentage of viable cells and necrosis compared to non-responders: for viable cells, this was respectively 10% vs. 30% when categorized by RECIST (p = .050) or respectively 22.50% vs. 50% when categorized with Choi (p = .015). For necrosis, this was 0% vs. 20% with RECIST (p < .001), Choi categories showed no significant differences (10% vs. 20%, p = .894). In contrast, fibrosis showed significant higher median values in responders when compared to non-responders. This was respectively 70% vs. 23% for RECIST (p = .005) and respectively 32.50% vs. 10% for Choi (p = .008).
3.6. Follow-up and outcome
Median FU since diagnosis was 42 months (IQR 24–68), with 57% of patients having no evidence of disease (NED) at the last FU moment. Eight patients developed an LR after primary disease (50% after R0, 50% after R1) with an LRFS of the median of 11 months (IQR 3–19), resulting in a 91% 5-year local control rate. DM occurred in 38 patients (almost all pulmonary metastasis), with a distant metastasis-free interval (DMFI) of the median of 17 months (IQR 8–22). The 5-year outcomes were 61.80% for DMFS and 61.70% for OS.
On univariate analysis, the only significant unfavorable predictor for LR was R1 resection (p = .007). Larger tumor size, high-grade tumors, and higher values of necrosis were significantly associated with a worse OS and DMFS on univariate analysis. After multivariate analysis, only tumor size and necrosis remained significant unfavorable prognostic factors for OS, for DMFS none remained significant. Fibrosis and viable cells were both on univariate and multivariate analysis not prognostic for the oncologic outcome. Multivariate analysis of OS and DMFS can be found in . Response evaluation as categorized by RECIST or Choi had no prognostic significance for LRFS, DMFS or OS when subdivided into responders vs. non-responders, nor when categorized following the four classic categories (CR, PR, SD, and PD).
Table 4. Multivariate analysis of tumor characteristics on the oncological outcome.
Discussion
In this study, radiological and histopathological response after nRT were evaluated and compared. In our cohort, radiological responders had a significantly lower median percentage of viable tumor cells, a higher median percentage of fibrosis, and interestingly lower median percentage of necrosis when compared to radiological non-responders, both on RECIST and Choi. Apart from necrosis, radiological nor other histopathological parameters were associated with oncologic outcomes.
The definition of histopathological response after nT has often been debated. A pathological response (pCR of pPR) in STS has been defined by means of a low percentage of viable cells [Citation6,Citation10–12], by means of a high percentage of necrosis [Citation11,Citation13–19], and/or by a high percentage of fibrosis [Citation6].
In several studies evaluating necrosis after neoadjuvant chemotherapy (nCT) for different bone sarcomas, a higher percentage of necrosis seems to be a prognostic factor for oncological outcome [Citation20–22]. In STS however, necrosis has shown conflicting results [Citation11,Citation13–19]. In a recently published large (n = 330) multicenter study, Bonvalot et al. evaluated pathological response after nT (majority after nRT n = 222), where pCR was defined as ≤5% viable cells or ≥95% necrosis [Citation11]. The radiological response was not described. Their results showed that pCR was associated with better oncological outcomes (DMFI, LRFS, DFS, and OS). In addition, a large meta-analysis including 21 studies with a total of 1663 mostly high-grade eSTS patients showed that <90% necrosis following nT (including both nRT and nCT) is a significant predictor for decreased DFS and OS [Citation23]. However, our study showed that a higher percentage of necrosis is associated with decreased OS. As few studies already have suggested, this could be explained by the hypothesis that necrosis is primary an inherent adverse tumor characteristic; (part of) the necrosis in STS is preexistent and an important denominator in determining the tumor grade (in which higher grades associated with the worse outcome) [Citation19,Citation24], which to date is indistinguishable from potential newly formed necrosis after treatment. nT thereby interferes with the determination of risk factors for recurrence by influencing diagnostic accuracy. In addition, no comparison was made of treatment-induced necrosis with initial necrosis on core biopsy at diagnosis, since necrosis on core biopsies is considered to be an inaccurate reflection of necrosis of the whole tumor.
Another potential definition of histopathological response could be measuring viable cells, with the proposal of the EORTC-STBSG evaluation score [Citation5]. However, this score system was evaluated in only one paper for prognostic value on a retrospective cohort of 100 patients after nRT, not demonstrating a prognostic value [Citation6]. Crombé et al. identified a significant lower risk for DMFS in case of <5% viable tumor cells after nCT [Citation12]. In our study, the percentage of viable cells was not prognostic for the outcome but did associate with the radiological response by both RECIST and Choi.
Schaefer et al. demonstrated that the percentage of fibrosis/hyalinization, rather than necrosis or viable cells (the EORTC-STBSG response score), is a prognostic factor for outcome in their study after nRT [Citation6]. In our data, fibrosis also showed higher median values for radiological responders (both RECIST and Choi) when compared with non-responders; however, there was no association with oncological outcome. Others have also suggested that fibrosis is a restorative response to newly formed necrosis that appears over time and that a combination of fibrosis and necrosis was a stronger predictive factor for the oncological outcome than fibrosis alone [Citation24,Citation25].
A combination of previously mentioned characteristics has already been implemented in a few studies to express histological response [Citation7,Citation9,Citation11,Citation26]. Concluding from these earlier considerations, a combination of viable cells and fibrosis as used by Stacchiotti et al. may be the best option; (low) viable cells should only be considered as a response if a minimum of 10% treatment-related changes are demonstrated (including fibrosis) [Citation7]. Necrosis (mostly preexistent) must be disregarded in these changes.nT has the advantage of being able to compare the radiological response with the histopathological response. The association between imaging and histopathology is very important since clinical decisions are often based on the interpretation of the radiological response. Traditionally, the radiological response was based on unidimensional measurements as applied in RECIST [Citation2]. These criteria were developed to provide uniform endpoints in clinical trials, especially in the metastatic/palliative setting when there are no histopathological response evaluations. In addition, the cutoff points in RECIST were chosen without substantiation. For example, in the nRT setting for STS, tumor shrinkage is a rare event except for MLS and some other rare subtypes [Citation16,Citation25,Citation27–30]. Furthermore, phenomena like treatment-induced cystic transformation, edema, and hemorrhage, can induce tumor expansion [Citation31], which is often called pseudoprogression like seen in immunotherapy-induced changes in different tumors. Not surprisingly, several studies have shown that reduction in tumor size in STS after nT (and therefore RECIST), poorly correlate with prognosis and histopathologic response [Citation25,Citation27,Citation32,Citation33]. The EORTC-STBSG group, therefore, does not recommend the use of size and volume as response predictor [Citation4]. In our study RECIST was not prognostic for an oncological outcome as well.
The Choi criteria were developed in the search for alternative assessment criteria [Citation3]. Several studies have already suggested that Choi might be a more reliable method for response evaluation than RECIST in STS [Citation7,Citation8,Citation34]. Neither of these scoring methods is prognostic for oncologic outcome in our study, but given the rare occurrence of tumor shrinkage, it seems logical to include more variables in response measurement criteria than tumor size alone. The EORTC-STBSG, therefore, recommends adding a diffusion-weighted MRI (DW-MRI) series in response assessment [Citation4]. DW-MRI series and its diffusion coefficient (ADC) estimates appear to have good reproducibility and are able to predict response [Citation35], where signs of response may be observed as early as two weeks after the start of treatment [Citation36]. Efforts are currently ongoing to develop a more optimal, uniform, and standardized imaging protocol [Citation4,Citation37–39].
Other imaging techniques have also been suggested for (early) response evaluation or even prediction of response, such as fluorodeoxyglucose positron-emission tomography (FDG-PET) or advanced imaging analysis such as radiomics [Citation40–43].
Of note, responses to radiotherapy varied widely between the different subtypes in our study. Both Choi and histopathological responses were mostly seen in MLS and UPS/NOS, while in other subtypes both radiological and histopathological response was very limited. An alternative might be response evaluation tailored per histological subtype, but this can only be evaluated in a study with enormous patient numbers. Allignet et al. elaborated on this and evaluated four histological subtypes of STS after nRT and described their subtype-specific responses [Citation10]. Radiosensitivity of MLS and synovial sarcoma (SS) were confirmed with a decrease in tumor size with mainly fibrosis in the resection specimen. This is consistent with the speculation that fibrosis might be of predictive value.
Whether histopathological response and/or radiological response evaluation after nCT can be compared to response evaluation after nRT (or vice versa) is not clear and previous studies have not clarified this yet. However, since there is no clear evidence that nRT improves overall survival, an effect on oncological outcomes such as DMFS and OS is most likely not expected after nRT.
This study is limited by its retrospective nature and the exclusion of several patients due to various reasons such as suboptimal MRI quality, missing imaging, or non-revisable histopathology. In addition, this study covers multiple histologic subtypes of STS. It is unclear whether response analysis can be performed uniformly for all STS or whether specific cut-off points are needed per histological subtype, since response per nT and/or per histological subtype may vary. A subtype-specific analysis has been recommended to perform [Citation4], given the heterogeneity of the various subtypes, but it was not feasible due to the sample size of our cohort. A third limitation is that the incidence of LR is very low, which is reflected in the excellent local control rates of nRT followed by resection but makes it difficult to identify prognostic factors with respect to LR. Future studies are needed to validate how both histological and imaging parameters can function as a true predictors of oncological outcomes.
Conclusion
RECIST, Choi, and the EORTC-STBSG response score show incongruent results in response evaluation after nRT for STS. The radiological response was significantly correlated with a lower percentage of viable cells and necrosis, but a higher percentage of fibrosis. Apart from necrosis, radiological nor other histopathological parameters were associated with oncologic outcomes. This study supports the potential role of fibrosis as a histopathological response characteristic. However, both in histopathology and radiology we were not able to demonstrate an unequivocal way to assess response, and we should therefore be cautious with assigning major clinical consequences to on-treatment response evaluation.
Supplemental Material
Download MS Word (17.3 KB)Supplemental Material
Download MS Word (33.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author (W.H.) upon reasonable request.
References
- Haas RL, Delaney TF, O'Sullivan B, et al. Radiotherapy for management of extremity soft tissue sarcomas: why, when, and where? Int J Radiat Oncol Biol Phys. 2012;84(3):572–580.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247.
- Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25(13):1753–1759.
- Messiou C, Bonvalot S, Gronchi A, et al. Evaluation of response after pre-operative radiotherapy in soft tissue sarcomas; the European Organisation for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC-STBSG) and Imaging Group recommendations for radiological examination and reporting with an emphasis on magnetic resonance imaging. Eur J Cancer. 2016;56:37–44.
- Wardelmann E, Haas RL, Bovee JV, et al. Evaluation of response after neoadjuvant treatment in soft tissue sarcomas; the European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC-STBSG) recommendations for pathological examination and reporting. Eur J Cancer. 2016;53:84–95.
- Schaefer IM, Hornick JL, Barysauskas CM, et al. Histologic appearance after preoperative radiation therapy for soft tissue sarcoma: assessment of the European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group response score. Int J Radiat Oncol Biol Phys. 2017;98(2):375–383.
- Stacchiotti S, Collini P, Messina A, et al. High-grade soft-tissue sarcomas: tumor response assessment–pilot study to assess the correlation between radiologic and pathologic response by using RECIST and Choi criteria. Radiology. 2009;251(2):447–456.
- Stacchiotti S, Verderio P, Messina A, et al. Tumor response assessment by modified Choi criteria in localized high-risk soft tissue sarcoma treated with chemotherapy. Cancer. 2012;118(23):5857–5866.
- Lansu J, Bovee J, Braam P, et al. Dose reduction of preoperative radiotherapy in myxoid liposarcoma: a nonrandomized controlled trial. JAMA Oncol. 2021;7(1):e205865.
- Allignet B, Meurgey A, Bouhamama A, et al. Impact of histological subtype on radiological and pathological response after neoadjuvant radiotherapy in soft tissue sarcoma. Eur J Surg Oncol. 2021;47(12):2995–3003.
- Bonvalot S, Wunder J, Gronchi A, et al. Complete pathological response to neoadjuvant treatment is associated with better survival outcomes in patients with soft tissue sarcoma: results of a retrospective multicenter study. Eur J Surg Oncol. 2021;47(8):2166–2172.
- Crombe A, Cousin S, Spalato-Ceruso M, et al. Implementing a machine learning strategy to predict pathologic response in patients with soft tissue sarcomas treated with neoadjuvant chemotherapy. JCO Clin Cancer Inform. 2021;5:958–972.
- Eilber FC, Rosen G, Eckardt J, et al. Treatment-induced pathologic necrosis: a predictor of local recurrence and survival in patients receiving neoadjuvant therapy for high-grade extremity soft tissue sarcomas. J Clin Oncol. 2001;19(13):3203–3209.
- Seldon C, Shrivastava G, Fernandez M, et al. Pathologic response rates after neoadjuvant therapy for sarcoma: a single institution study. Cancers. 2021;13(5):1074.
- Shah D, Borys D, Martinez SR, et al. Complete pathologic response to neoadjuvant radiotherapy is predictive of oncological outcome in patients with soft tissue sarcoma. Anticancer Res. 2012;32(9):3911–3915.
- Canter RJ, Martinez SR, Tamurian RM, et al. Radiographic and histologic response to neoadjuvant radiotherapy in patients with soft tissue sarcoma. Ann Surg Oncol. 2010;17(10):2578–2584.
- Mullen JT, Hornicek FJ, Harmon DC, et al. Prognostic significance of treatment-induced pathologic necrosis in extremity and truncal soft tissue sarcoma after neoadjuvant chemoradiotherapy. Cancer. 2014;120(23):3676–3682.
- Lucas DR, Kshirsagar MP, Biermann JS, et al. Histologic alterations from neoadjuvant chemotherapy in high-grade extremity soft tissue sarcoma: clinicopathological correlation. Oncologist. 2008;13(4):451–458.
- Gannon NP, Stemm MH, King DM, et al. Pathologic necrosis following neoadjuvant radiotherapy or chemoradiotherapy is prognostic of poor survival in soft tissue sarcoma. J Cancer Res Clin Oncol. 2019 May;145(5):1321–1330.
- Picci P, Bohling T, Bacci G, et al. Chemotherapy-induced tumor necrosis as a prognostic factor in localized Ewing’s sarcoma of the extremities. J Clin Oncol. 1997;15(4):1553–1559.
- Vasquez L, Tarrillo F, Oscanoa M, et al. Analysis of prognostic factors in High-Grade osteosarcoma of the extremities in children: a 15-year single-institution experience. Front Oncol. 2016;6:22.
- Bacci G, Longhi A, Versari M, et al. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer. 2006;106(5):1154–1161.
- Salah S, Lewin J, Amir E, et al. Tumor necrosis and clinical outcomes following neoadjuvant therapy in soft tissue sarcoma: a systematic review and meta-analysis. Cancer Treat Rev. 2018;69:1–10.
- Cates JMM. Histologic response to neoadjuvant therapy is not predictive of favorable outcomes in high-grade pleomorphic soft tissue sarcoma. Am J Surg Pathol. 2019;43(4):564–572.
- Roberge D, Skamene T, Nahal A, et al. Radiological and pathological response following pre-operative radiotherapy for soft-tissue sarcoma. Radiother Oncol. 2010;97(3):404–407.
- Bonvalot S, Rutkowski PL, Thariat J, et al. NBTXR3, a first-in-class radioenhancer hafnium oxide nanoparticle, plus radiotherapy versus radiotherapy alone in patients with locally advanced soft-tissue sarcoma (Act.In.Sarc): a multicentre, phase 2-3, randomised, controlled trial. Lancet Oncol. 2019;20(8):1148–1159.
- Le Grange F, Cassoni AM, Seddon BM. Tumour volume changes following pre-operative radiotherapy in borderline resectable limb and trunk soft tissue sarcoma. Eur J Surg Oncol. 2014;40(4):394–401.
- Betgen A, Haas RL, Sonke JJ. Volume changes in soft tissue sarcomas during preoperative radiotherapy of extremities evaluated using cone-beam CT. J Radiat Oncol. 2013;2(1):55–62.
- de Vreeze RS, de Jong D, Haas RL, et al. Effectiveness of radiotherapy in myxoid sarcomas is associated with a dense vascular pattern. Int J Radiat Oncol Biol Phys. 2008;72(5):1480–1487.
- Pitson G, Robinson P, Wilke D, et al. Radiation response: an additional unique signature of myxoid liposarcoma. Int J Radiat Oncol Biol Phys. 2004;60(2):522–526.
- Walker EA, Salesky JS, Fenton ME, et al. Magnetic resonance imaging of malignant soft tissue neoplasms in the adult. Radiol Clin North Am. 2011;49(6):1219–1234, vi.
- Look Hong NJ, Hornicek FJ, Harmon DC, et al. Neoadjuvant chemoradiotherapy for patients with high-risk extremity and truncal sarcomas: a 10-year single institution retrospective study. Eur J Cancer. 2013;49(4):875–883.
- Grunwald V, Litiere S, Young R, et al. Absence of progression, not extent of tumour shrinkage, defines prognosis in soft-tissue sarcoma – An analysis of the EORTC 62012 study of the EORTC STBSG. Eur J Cancer. 2016;64:44–51.
- Taieb S, Saada-Bouzid E, Tresch E, et al. Comparison of response evaluation criteria in solid tumours and Choi criteria for response evaluation in patients with advanced soft tissue sarcoma treated with trabectedin: a retrospective analysis. Eur J Cancer. 2015;51(2):202–209.
- Winfield JM, Tunariu N, Rata M, et al. Extracranial soft-tissue tumors: repeatability of apparent diffusion coefficient estimates from diffusion-weighted MR imaging. Radiology. 2017;284(1):88–99.
- Meyer JM, Perlewitz KS, Hayden JB, et al. Phase I trial of preoperative chemoradiation plus sorafenib for high-risk extremity soft tissue sarcomas with dynamic contrast-enhanced MRI correlates. Clin Cancer Res. 2013;19(24):6902–6911.
- Crombe A, Le Loarer F, Cornelis F, et al. High-grade soft-tissue sarcoma: optimizing injection improves MRI evaluation of tumor response. Eur Radiol. 2019;29(2):545–555.
- Costa FM, Martins PH, Canella C, et al. Multiparametric MR imaging of soft tissue tumors and pseudotumors. Magn Reson Imaging Clin N Am. 2018;26(4):543–558.
- Gennaro N, Reijers S, Bruining A, et al. Imaging response evaluation after neoadjuvant treatment in soft tissue sarcomas: where do we stand? Crit Rev Oncol Hematol. 2021;160:103309.
- Kasper B, Hohenberger P, Strauss LG, et al. The use of fluorine-18 fluorodesoxyglycose-positron emission tomography for treatment monitoring in patients with soft tissue sarcomas. Hell J Nucl Med. 2010;13(1):40–44.
- Herrmann K, Benz MR, Czernin J, et al. 18F-FDG-PET/CT imaging as an early survival predictor in patients with primary high-grade soft tissue sarcomas undergoing neoadjuvant therapy. Clin Cancer Res. 2012;18(7):2024–2031.
- Peeken JC, Neumann J, Asadpour R, et al. Prognostic assessment in high-grade soft-tissue sarcoma patients: a comparison of semantic image analysis and radiomics. Cancers. 2021;13(8):1929.
- Nardone V, Boldrini L, Grassi R, et al. Radiomics in the setting of neoadjuvant radiotherapy: a new approach for tailored treatment. Cancers. 2021;13(14):3590.
