Introduction
In 1977, Esterly et al. coined the term ‘eruptive vellus hair cysts’ (EVHC) to describe an idiopathic, persistent, hyperpigmented, monomorphous, papular eruption observed in two children. On pathology, cysts in the mid-dermis were noted to contain multiple fragmented vellus hair shafts. There have since been nearly 130 cases of EVHC reported in the literature [Citation1].
EVHC typically presents as a 1–7 mm symmetrical, monomorphous, skin-colored, and dome-shaped eruption of papules that can be grouped or diffuse. The eruption can present anywhere on the body but has a predilection for the anterior chest, abdomen, upper limbs, and face and may be congenital or acquired [Citation2–4]. Most cases are idiopathic, persistent, and asymptomatic [Citation4]. While the exact mechanism underlying EVHC pathogenesis is unknown, it is thought that a developmental issue in vellus hair follicles causes infundibular occlusion, leading to cystic dilation, hair retention, and hair bulb atrophy [Citation1]. Histologically, the cysts are lined by a stratified squamous epithelium in the top or middle dermis, may contain keratin, and are characterized by the presence of obliquely cut vellus hair shafts. Patients with EVHC tend to seek treatment for cosmetic concerns, and most therapies rely on trials of retinoic acid, surgical removal, or laser with varying results [Citation4]. Here, with consent obtained from the patient, we report a case of a patient who developed EVHC during radiation therapy (RT) for breast cancer. Based on a comprehensive literature review, only eight cases of EVHC have presented after a potential trigger, with the rest considered idiopathic or familial. To our knowledge, this is the first case of EVHC arising after any type of cancer treatment, making it an important cutaneous side effect for dermatologists and oncologists to consider when encountering a similar cutaneous eruption during RT.
Case report
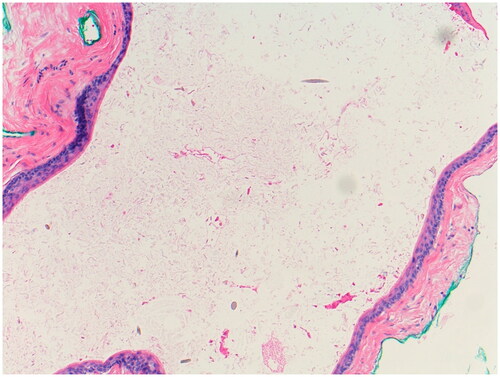
A 39-year-old woman presented to the oncodermatology clinic for a pruritic, diffuse rash during RT for left breast cancer. During week four of RT (after a cumulative dose of 42 Gy), she developed a new, generalized, cutaneous eruption that appeared similar to ‘insect bites’, per her radiation oncologist. The lesions did not resolve after her RT course was completed. She presented to the oncodermatology clinic two weeks after completion of her RT course. Other than her recent diagnosis of breast cancer treated with lumpectomy, sentinel node biopsy, and RT, her medical history was notable only for Breast Cancer Gene-2 (BRCA-2) positivity. The patient denied any previous dermatologic history, use of new medications, or use of over-the-counter topicals. She did not receive chemotherapy or hormonal therapy. Physical exam at that time showed dozens of subcutaneous nodules with overlying excoriations and hyperpigmentation over the irradiated field (left breast) and extending to the trunk and thighs (). The differential diagnosis included eruptive lipomas, steatocystomas, vellus hair cysts, and cutaneous metastases. A punch biopsy of a lesion on the patient’s right buttock revealed a cyst lined by infundibular epithelium and filled with orthokeratotic cornified cells. Fragments of small hair shafts found within the dermis led to a conclusive diagnosis of eruptive vellus hair cysts (). The patient considered surgical excision of lesions but did not return for follow-up. The eruption persisted for at least nine months after the initial presentation.
Figure 1. (A-B) Clinical Photographs of Eruptive Vellus Hair Cysts Subcutaneous nodules with overlying excoriations and hyperpigmentation on the trunk (A) and thighs (B).
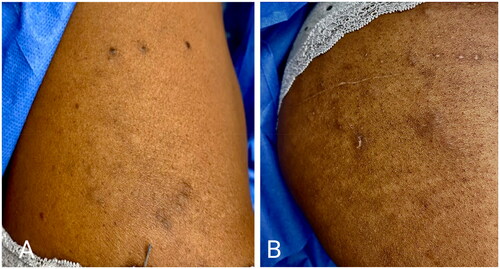
Figure 2. Histology of Eruptive Vellus Hair Cyst Cyst within the dermis with numerous vellus hair cysts in the lumen (Hematoxylin & Eosin, 100x).
To our knowledge, this is the first case of EVHC arising in the setting of cancer therapy, and specifically as a sequela to RT. In fact, in most of the described cases published in the literature, EVHC appeared without a triggering event, and rarely were any cases described in the setting of medical treatment. While EVHC are benign, they can be symptomatic and cosmetically distressing. Due to their rarity, lack of a clear triggering event, and absence of standardized treatment, they may present a clinical challenge. Below we will review the literature describing the clinical presentations of EVHC to highlight their variability and treatment challenges in the hopes of promoting further research into their pathogenesis and management.
Discussion
We present the first case of a persistent EVHC eruption triggered by RT. This eruption was generalized, as it was not limited to the irradiated field. The phenomenon of a new skin eruption due to RT, extending beyond or not involving the irradiated field at all, has been well described in other cutaneous conditions, such as post-irradiation morphea [Citation5,Citation6], eosinophilic polymorphic and pruritic eruption associated with radiotherapy [Citation7], generalized bullous pemphigoid [Citation8], radiotherapy-induced pemphigus [Citation9], erythema multiforme or erythema-multiforme-like rash [Citation10], and RT-induced polymorphous light eruption [Citation10]. No particular time frame or radiation dose has been implicated in these eruptions. Like the present case, breast cancer was the most common cancer associated with these generalized eruptions, portending a potential common hormonal pathogenic link between breast cancer and resulting radiation-induced generalized cutaneous reactions, as suggested by some authors [Citation7]. Furthermore, some theorize that EVHC arise due to a defect in keratinization or vellus hair follicle formation, both of which can be heavily influenced by sex-hormone alterations [Citation11–14]. Another hypothesis for radiation-induced generalized cutaneous reactions is that localized RT may induce a systemic release of autoantibodies, antigens, immune complexes, or cytokines, which could trigger widespread cutaneous reactions [Citation5,Citation15]. As the pathogenesis of EVHC itself is poorly understood, it is difficult to clearly explicate how RT might induce this eruption, but the current literature supports and provides significant evidence for generalized radiation-induced dermatologic conditions with varying known etiologies. Additionally, the time frame of this patient’s eruption (during RT and persisting afterward), along with a lack of comorbidities associated with EVHC (such as steatocystoma), or a contributory family history, supports our hypothesis.
As EVHC are rare, we performed a review of the literature to better clarify their typical presentation to aid in diagnosis. We reviewed all the English articles (n = 76) describing EVHC cases in the PubMed database. From the 76 reports, 121 individual cases of EVHC were described. Epidemiologically, EVHC were slightly more frequent in females than males (50.4% versus 44.6%) and most commonly appeared between the ages of 1–20 years old (54.5%) with a mean age of 18.4 years old at onset. Most of the reported cases (62.0%) did not disclose the race/ethnicity of the patients with EVHC, but of those reported, the highest frequency of cases occurred in Caucasians (13.2%) [Citation16–24], followed by Blacks/African Americans (6.6%) [Citation25–30] and Japanese (6.6%) [Citation31–37]. Although EVHC is traditionally considered to occur more frequently in patients with a family history, 80.2% of cases had no known genetic predisposition or family history, while 19.8% of cases presented with a family history of EVHC [Citation2,Citation3,Citation17,Citation18,Citation23,Citation38–46].
Regarding the clinical course of EVHC, 86% of cases presented asymptomatically, while 14% reported associated symptoms, primarily pruritus [Citation2,Citation18,Citation22,Citation25,Citation26,Citation30,Citation31,Citation40,Citation41,Citation45,Citation47–50]. Upon physical exam, EVHC most commonly appeared as multiple, discrete, dome-shaped, hyper-pigmented or flesh-colored, soft, or cystic papules. The most commonly considered differential diagnoses reported were keratosis pilaris (11.6%) [Citation24,Citation45,Citation51–53], acneiform eruptions (10.7%) [Citation45,Citation52,Citation53], perforating dermatoses (10.7%) [Citation45,Citation52,Citation53], folliculitis (9.1%) [Citation45], and acne vulgaris (5.0%) [Citation2,Citation25,Citation34,Citation54,Citation55]. 6.6% of cases reported a potential trigger for EVHC. The proposed triggers included scratching/mechanical trauma (n = 4) [Citation55–58], dialysis (n = 2) [Citation34], molluscum contagiosum treated with imiquimod [Citation19], and 3% minoxidil application [Citation59]. In 15.4% of cases, other conditions were associated with the presentation of EVHC, most frequently steatocystoma (5.8%) [Citation35,Citation36,Citation40,Citation44,Citation60–62], chronic kidney disease (1.7%) [Citation34], ectodermal dysplasia (1.7%) [Citation16,Citation27], milia (1.7%) [Citation19,Citation44], and trichostasis spinulosa (1.7%) [Citation11,Citation24].
Clinicians most frequently treated EVHC with a retinol-derived topical (9.1%) [Citation11,Citation16,Citation21,Citation24–26,Citation38,Citation63–67], surgical excision/extraction with or without electrocauterization (5.8%) [Citation19,Citation38,Citation47,Citation59,Citation68,Citation69], carbon dioxide (CO2) laser (4.1%) [Citation46,Citation70–72], erbium-doped yttrium aluminum garnet (Er:YAG) laser (3.3%) [Citation22,Citation50,Citation67,Citation73], oral vitamin A or vitamin A derivatives (2.5%) [Citation17,Citation23,Citation27], or topical lactic acid (1.7%) [Citation23,Citation30]. Most cases did not report subsequent treatment or spontaneous resolution (68.7%). However, some patients underwent spontaneous resolution without treatment (3.31%) [Citation12,Citation45,Citation74,Citation75]. 19 cases (15.7%) resolved upon treatment [Citation1,Citation11,Citation19,Citation21–23,Citation30,Citation46,Citation50,Citation55,Citation59,Citation66,Citation68–71,Citation76], while 9 treated cases (7.4%) resulted in no improvement [Citation16,Citation17,Citation25–27,Citation38,Citation47,Citation63,Citation64]. The most effective treatments were topical lactic acid (2/2, 100%) [Citation23,Citation30], CO2 laser (4/5, 80%) [Citation46,Citation70,Citation71], Er:YAG laser (3/4, 75%) [Citation22,Citation50], surgical extraction (5/7, 71.4%) [Citation19,Citation59,Citation68,Citation69,Citation76], and retinol-derived topicals (3/11, 27.3%) [Citation11,Citation21,Citation66].
A comprehensive review of the literature demonstrates that EVHC can present in an epidemiologically heterogeneous population with variable family histories, triggers, disease courses, disease associations, and responses to treatment. This non-uniform presentation can make it difficult for clinicians to recognize or consider when encountering an unknown cutaneous eruption. Our goal in presenting this case is to expose RT as a newly recognized trigger for the development of EVHC. Additionally, while EVHC may be cosmetically concerning, it is benign and therefore likely does not necessitate ceasing or reducing RT. This case is valuable as it broadens the collection of known cutaneous effects of RT, as well as widens the differential for dermatologists and oncologists encountering a similar eruption after RT.
Supplemental Material
Download PDF (573.9 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data pertaining to the results of this report are available as part of the report and no additional sources of data are required.
References
- Esterly NB, Fretzin DF, Pinkus H. Eruptive vellus hair cysts. Arch Dermatol. 1977;113(4):500–503.
- Yaremkevych R, Andrashko Y, Zimenkovskyi A, et al. An alternative diagnostic method of eruptive vellus hair cysts: report of a familial case with pruritus. Dermatol Ther. 2020;33(1):e13147.
- Jerdan K, St Claire K, Bain M. Eruptive vellus hair cysts in identical triplets with dermoscopic findings. Cutis. 2018;102(5):367–369.
- Torchia D, Vega J, Schachner LA. Eruptive vellus hair cysts: a systematic review. Am J Clin Dermatol. 2012;13(1):19–28.
- Kushi J, Csuka ME. Generalized morphea after breast cancer radiation therapy. Case Rep Rheumatol. 2011;2011:951948.
- Spalek M, Jonska-Gmyrek J, Gałecki J. Radiation-induced morphea – a literature review. J Eur Acad Dermatol Venereol. 2015;29(2):197–202.
- García-Donoso C, Tardío JC, Arias D, et al. Eosinophilic, polymorphic and pruritic eruption associated with radiotherapy (EPPER) in two patients with breast tumour. J Eur Acad Dermatol Venereol. 2007;21(8):1102–1104.
- Nguyen T, Kwan JM, Ahmed AR. Relationship between radiation therapy and bullous pemphigoid. Dermatology. 2014;229(2):88–96.
- Badri T, Hammami H, Lachkham A, et al. Radiotherapy-induced pemphigus vulgaris with autoantibodies targeting a 110 kDa epidermal antigen. Int J Dermatol. 2011;50(12):1475–1479.
- Veness MJ, Dwyer PK. Erythema multiforme-like reaction associated with radiotherapy. Australas Radiol. 1996;40(3):334–337.
- Lazarov A, Amichai B, Cagnano M, et al. Coexistence of trichostasis spinulosa and eruptive vellus hair cysts. Int J Dermatol. 1994;33(12):858–859.
- Lee S, Kim JG. Eruptive vellus hair cyst. Clinical and histologic findings. Arch Dermatol. 1979;115(6):744–746.
- Grymowicz M, Rudnicka E, Podfigurna A, et al. Hormonal effects on hair follicles. Int J Mol Sci. 2020;21(15):5342.
- Gratton R, Del Vecchio C, Zupin L, et al. Unraveling the role of sex hormones on keratinocyte functions in human inflammatory skin diseases. Int J Mol Sci. 2022;23(6):3132.
- Robertson JM, Clarke DH, Pevzner MM, et al. Breast conservation therapy. Severe breast fibrosis after radiation therapy in patients with collagen vascular disease. Cancer. 1991;68(3):502–508.
- Köse O, Taştan HB, Deveci S, et al. Anhidrotic ectodermal dysplasia with eruptive vellus hair cysts. Int J Dermatol. 2001;40(6):401–402.
- Stiefler RE, Bergfeld WF. Eruptive vellus hair cysts–an inherited disorder. J Am Acad Dermatol. 1980;3(4):425–429.
- Piepkorn MW, Clark L, Lombardi DL. A kindred with congenital vellus hair cysts. J Am Acad Dermatol. 1981;5(6):661–665.
- Waldemer-Streyer RJ, Jacobsen E. A tale of two cysts: steatocystoma multiplex and eruptive vellus hair Cysts-Two case reports and a review of the literature. Case Rep Dermatol Med. 2017;2017:3861972.
- Armstrong CR, Yeager JK, Vidmar DA. Multiple papulocystic lesions on the trunk. Eruptive vellus hair cysts (EVHC). Arch Dermatol. 1995;131(3):343–346.
- Morgan MB, Kouseff BG, Silver A, et al. Eruptive vellus hair cysts and neurologic abnormalities: two related conditions? Cutis. 1991;47(6):413–415.
- Kageyama N, Tope WD. Treatment of multiple eruptive hair cysts with erbium: YAG laser. Dermatol Surg. 1999;25(10):819–822.
- Mayron R, Grimwood RE. Familial occurrence of eruptive vellus hair cysts. Pediatr Dermatol. 1988;5(2):94–96.
- Young MC, Jorizzo JL, Sanchez RL, et al. Trichostasis spinulosa. Int J Dermatol. 1985;24(9):575–580.
- Binhlam JQ, Gross AS, Onadeko OO, et al. Acneiform eruption due to eruptive vellus hair cysts. South Med J. 1992;85(3):322–325.
- Reep MD, Robson KJ. Eruptive vellus hair cysts presenting as multiple periorbital papules in a 13-year-old boy. Pediatr Dermatol. 2002;19(1):26–27.
- Romiti R, Festa Neto C. Eruptive vellus hair cysts in a patient with ectodermal dysplasia. J Am Acad Dermatol. 1997;36(2 Pt 1):261–262.
- Burns DA, Calnan CD. Eruptive vellus hair cysts. Clin Exp Dermatol. 1981;6(2):209–213.
- Sina B, Burnett JW. Eruptive vellus hair cysts. Cutis. 1984;33(5):503–504.
- Tran B, Curtis AR, Lee AD, et al. Acquired eruptive vellus hair cysts. Int J Dermatol. 2011;50(8):1032–1033.
- Utsunomiya N, Oyama N, Chino T, et al. Multiple subcutaneous cholesterol granulomas arising in eruptive vellus hair cysts: a case report and published work review of 11 cases. J Dermatol. 2017;44(4):481–482.
- Oiso N, Matsuda H, Kawada A. Eruptive vellus hair cysts of the labia majora: detection of openings of the cysts to the epidermis by dermoscopy. Eur J Dermatol. 2013;23(3):417–418.
- Okamoto H, Imamura S. Eruptive vellus hair cysts in a japanese adult. Arch Dermatol. 1982;118(8):537–538.
- Mieno H, Fujimoto N, Tajima S. Eruptive vellus hair cyst in patients with chronic renal failure. Dermatology. 2004;208(1):67–69.
- Ohtake N, Kubota Y, Takayama O, et al. Relationship between steatocystoma multiplex and eruptive vellus hair cysts. J Am Acad Dermatol. 1992;26(5 Pt 2):876–878.
- Ogawa Y, Nogita T, Kawashima M. The coexistence of eruptive vellus hair cysts and steatocystoma multiplex. J Dermatol. 1992;19(9):570–571.
- Nogita T, Chi HI, Nakagawa H, et al. Eruptive vellus hair cysts with sebaceous glands. Br J Dermatol. 1991;125(5):475–476.
- Pauline G, Alain H, Jean-Jaques R, et al. Eruptive vellus hair cysts: an original case occurring in twins. Int J Dermatol. 2015;54(6):e209-12–e212.
- Ponzo MG, Van Allen MI, Armstrong L, et al. Case series: a kindred with eruptive vellus hair cysts and systemic features. J Cutan Med Surg. 2017;21(6):564–567.
- Bridges AG, Erickson LA. Co-occurrence of steatocystoma multiplex, eruptive vellus hair cysts, and trichofolliculomas. Cutis. 2017;100(1):E23–E26.
- Rodgers SA, Kitagawa K, Selim MA, et al. Familial eruptive vellus hair cysts. Pediatr Dermatol. 2012;29(3):367–369.
- Chan KH, Tang WY, Lam WY, et al. Eruptive vellus hair cysts presenting as bluish-grey facial discoloration masquering as naevus of ota. Br J Dermatol. 2007;157(1):188–189.
- Sardy M, Karpati S. Needle evacuation of eruptive vellus hair cysts. Br J Dermatol. 1999;141(3):594–595.
- Patrizi A, Neri I, Guerrini V, et al. Persistent milia, steatocystoma multiplex and eruptive vellus hair cysts: variable expression of multiple pilosebaceous cysts within an affected family. Dermatology. 1998;196(4):392–396.
- Lee S, Kim JG, Kang JS. Eruptive vellus hair cysts. Arch Dermatol. 1984;120(9):1191–1195.
- Huerter CJ, Wheeland RG. Multiple eruptive vellus hair cysts treated with carbon dioxide laser vaporization. J Dermatol Surg Oncol. 1987;13(3):260–263.
- Watson A. Eruptive vellus hair cysts. Int J Dermatol. 1982;21(5):273–274.
- Choi R, MacLean KD, Davidson HC, et al. Vellus hair cyst of the orbit. Ophthalmic Plast Reconstr Surg. 2017;33(3S Suppl 1):S89–S91.
- Zaharia D, Kanitakis J. Eruptive vellus hair cysts: report of a new case with immunohistochemical study and literature review. Dermatology. 2012;224(1):15–19.
- Helbig D, Bodendorf MO, Grunewald S, et al. Comparative treatment of multiple vellus hair cysts with the 2940 nm Er: YAG and 1540 nm Er: glass laser. J Cosmet Laser Ther. 2011;13(5):223–226.
- Somer M, Peippo M, Aalto-Korte K, et al. Cardio-facio-cutaneous syndrome: three additional cases and review of the literature. Am J Med Genet. 1992;44(5):691–695.
- Benoldi D, Allegra F. Congenital eruptive vellus hair cysts. Int J Dermatol. 1989;28(5):340–341.
- d‘Aubermont PC, Honig PJ, Wood MG. Eruptive vellus hair cysts: a benign condition. J Pediatr. 1982;101(5):727–730.
- Woudenberg YJ, Lucas C, Latour C, et al. Acceptance of insulin therapy: A long shot? Psychological insulin resistance in primary care. Diabet Med. 2012;29(6):796–802.
- Erkek E, Kurtipek GS, Duman D, et al. Eruptive vellus hair cysts: report of a pediatric case with partial response to calcipotriene therapy. Cutis. 2009;84(6):295–298.
- Lew BL, Lee MH, Haw CR. Unilateral eruptive vellus hair cysts occurring on the face. J Eur Acad Dermatol Venereol. 2006;20(10):1314–1316.
- Park JH, Her Y, Chun BM, et al. A case of eruptive vellus hair cysts that developed on the labium major. Ann Dermatol. 2009;21(3):294–296.
- Cheng H, Sohal S, Cheung K. Eruptive vellus hair cysts of the vulva. Australas J Dermatol. 2017;58(4):e254–e255.
- Eun DH, Kim SM, Jang YH, et al. Facial eruptive vellus hair cysts occurred after 3% minoxidil application. Ann Dermatol. 2018;30(1):95–96.
- Arceu M, Martinez G, Alfaro D, et al. Ultrasound morphologic features of steatocystoma multiplex with clinical correlation. J Ultrasound Med. 2020;39(11):2255–2260.
- Yoshida M, Oiso N, Kurokawa I, et al. A case of multiple pilosebaceous cysts. Case Rep Dermatol. 2010;2(2):116–119.
- Plewig G. Eruptive vellus hair cysts. A follicular cyst of the sebaceous duct (sometimes). Am J Dermatopathol. 1990;12(5):538–539.
- Held JL, Andrew JE, Toback AC. Eruptive vellus hair cysts. Cutis. 1987;40(3):259–260.
- Karadag AS, Cakir E, Pelitli A. Eruptive vellus hair cysts: an alternative diagnosing method. Indian J Dermatol Venereol Leprol. 2009;75(5):537–538.
- Khatu S, Vasani R, Amin S. Eruptive vellus hair cyst presenting as asymptomatic follicular papules on extremities. Indian Dermatol Online J. 2013;4(3):213–215.
- Fisher DA. Retinoic acid in the treatment of eruptive vellus hair cysts. J Am Acad Dermatol. 1981;5(2):221–222.
- Saks K, Levitt JO. Tazarotene 0.1 percent cream fares better than erbium: YAG laser or incision and drainage in a patient with eruptive vellus hair cysts. Dermatol Online J. 2006;12(6):7.
- Kaya TI, Tataroglu C, Tursen U, et al. Eruptive vellus hair cysts: an effective extraction technique for treatment and diagnosis. J Eur Acad Dermatol Venereol. 2006;20(3):264–268.
- Jakobiec FA, Stagner AM, Freitag SK, et al. Unusual eyelid dermal keratinous cysts of pilosebaceous origin. Ophthalmic Plast Reconstr Surg. 2016;32(2):93–97.
- Fernandez-Torres R, Del Pozo J, Castineiras I, et al. Treatment of multiple eruptive vellus hair cysts with carbon dioxide laser vaporization and manual lateral pressure. Clin Exp Dermatol. 2009;34(8):e716–8.
- Zhu Q, Huang Y, Cui X, et al. Eruptive vellus hair cysts diagnosed using dermatological imaging technique. Australas J Dermatol. 2021;62(1):86–88.
- Shi G, Zhou Y, Cai YX, et al. Clinicopathological features and expression of four keratins (K10, K14, K17 and K19) in six cases of eruptive vellus hair cysts. Clin Exp Dermatol. 2014;39(4):496–499.
- Coras B, Hohenleutner U, Landthaler M, et al. Early recurrence of eruptive vellus hair cysts after Er: YAG laser therapy: case report and review of the literature. Dermatol Surg. 2005;31(12):1741–1744.
- Kwon KS, Lee HT, Jang HS, et al. A case of generalized eruptive vellus hair cysts. J Dermatol. 1997;24(8):556–557.
- Grattan CE, Harman RR. Eruptive vellus hair cysts. Bristol Med Chir J. 1986;101(1):10–11.
- Papakonstantinou E, Franke I, Gollnick H. Facial steatocystoma multiplex combined with eruptive vellus hair cysts: a hybrid? J Eur Acad Dermatol Venereol. 2015;29(10):2051–2053.
