Abstract
Background
Current guidelines in HER2-positive metastatic breast cancer (mBC) recommend the combination of trastuzumab and a chemotherapeutic agent for 3rd line or later treatments. This study aims to describe the treatment of HER2-positive mBC in 3rd line or later after previous treatment with T-DM1 for mBC in a real-world setting.
Material and methods
This observational population-based study included all women diagnosed with HER2-positive mBC in Denmark, previously treated with T-DM1 in the metastatic setting. Patients were included on the date of progression leading to initiation of 3rd line treatment if the patient had received T-DM1 in 1st or 2nd line. If the patient received T-DM1 in 3rd line or later the inclusion was based on the date of progression on T-DM1. The primary end points were overall survival (OS) and progression-free survival (PFS).
Results
The study included 272 women with a mean age of 59 (27–86) and a median of 3 (2–11) treatment lines prior to inclusion. At index, all patients had received T-DM1 and 167 (62%) patients had received pertuzumab in the metastatic setting. During follow-up 183 patients received chemotherapy. Of these patients, 120 received chemotherapy combined with trastuzumab, 50 received chemotherapy combined with other HER2-targeted therapy, and 13 received chemotherapy as monotherapy. The remaining 89 patients received either HER2-targeted monotherapy (41), endocrine therapy (31), experimental treatment (10), or no treatment (7). Median PFS was 5.5 months (95% CI, 4.8–6.5) and median OS was 18.5 months (95% CI, 16.2–21.3).
Conclusion
In this real-world study, we found that patients were treated with a wide variety of anti-cancer agents with modest efficacy. However, patients in this study did not have access to newer therapies like tucatinib and T-DXd.
Introduction
Approximately 20% of women diagnosed with breast cancer show an overexpression or amplification of Human epidermal growth factor receptor 2 (HER2) [Citation1,Citation2]. HER2-positive breast cancer has historically been associated with an aggressive course, a high recurrence rate, and a disposition for brain metastases [Citation3]. HER2-positive mBC is associated with an increased risk of developing metastases in the central nervous system (CNS). Previous reports have shown that up to 50% of patients with HER2-positive mBC develop brain metastases during the course of their disease with a median time from diagnosis to development of CNS metastases of 76.2 months [Citation3]. Patients with CNS metastases have been shown to have a lower median OS than patients without CNS metastases (20.8 vs 46.7 months) [Citation3].
New anti-HER2 targeted therapies have improved clinical outcome and prolonged survival, with dual blockade (trastuzumab, pertuzumab) plus a taxane being the standard 1st line treatment and trastuzumab emtansine (T-DM1) being the standard 2nd line treatment. After receiving 1st and 2nd line treatment for HER2-positive metastatic breast cancer (mBC) there is currently no standard treatment for 3rd line. Danish guidelines recommend continued HER2-targeted therapy (often trastuzumab) combined with chemotherapy. Thus, the chosen chemotherapeutic agent should be individualized and based on toxicity and patient preferences.
Commonly used 3rd line therapies include trastuzumab combined with capecitabine and lapatinib combined with trastuzumab. These regimens have shown response rates between 10% to 22% in later lines of treatment [Citation4,Citation5]. The low response rate in subsequent treatment lines highlights an unmet need for new treatment options for patients with disease progression following the failure of the dual blockade and T-DM1. Currently, no standard of care treatment in the 3rd line exists, but results from trials with trastuzumab deruxtecan (DESTINY-Breast01 and DESTINY-Breast03), tucatinib (HER2CLIMB) and neratinib (NALA) have been promising [Citation6–9]).
To our knowledge, no real-world, nationwide study has described how patients are treated in 3rd line or later in the metastatic setting for HER2-positive breast cancer. Furthermore, there is a lack of evidence describing outcomes for different treatment modalities in a nationwide population. This Danish observational retrospective real-world study was conducted to describe patient characteristics, treatment patterns, and outcomes for patients in 3rd line or later in HER2-positive mBC. As the landscape of treatment for HER2-positive mBC is changing rapidly this study provides a clear view of the effect of present and older treatment modalities for HER2-positive mBC. Knowledge of the treatment patterns used by physicians and the efficacy of these treatments are essential for evaluating new treatment options in the future. Both economic and efficacy analyses depend on this kind of real-world data when considering the implementation of new and often very expensive treatments.
Methods
Study design
The study is observational, nation-wide, and population-based. The data collection is retrospective and thus non-interventional including all departments of oncology in Denmark utilizing the Danish Breast Cancer Group (DBCG) database.
Patient selection criteria and population
All included patients were diagnosed with HER2-positive metastatic breast cancer and were previously treated with T-DM1 in the metastatic setting. The patients were treated in 3rd line or later in the metastatic setting with an index date between January 1st, 2014 to December 31st, 2019. The index date is the progression date leading to subsequent initiation of 3rd-line treatment if the patient received T-DM1 in 1st or 2nd line. If the patient received T-DM1 in 3rd line or later the index date was the date of progression on T-DM1.
Data source
The nationwide, population-based clinical DBCG database used in this study includes data on demographics, diagnosis, treatment patterns, pathology, and follow-up. Prospectively collected data from clinical practice concerning primary diagnosis and treatment was extracted. Retrospectively collected data concerning metastatic disease included date of disease progression, location of metastases, treatment modalities with start and end date, and reason for discontinuation.
Objectives
The study objectives were to describe characteristics at the index and according to the presence of CNS metastases.
Further, to describe treatment patterns from the index date, with the six most used treatments in the line following inclusion and a 7th category for all other treatment modalities being reported. Finally, to describe clinical outcomes progression-free survival (PFS), time on treatment (ToT), and overall survival (OS), per treatment line and per treatment modality, all stratified by the presence CNS metastases.
End points
The primary endpoints were OS and PFS. The time scale defining the risk set was time since the index date and end of follow-up was March 1st, 2021. No patients were lost to follow-up
OS was estimated from the index date until death from any cause or end of follow-up, whichever occurred first. For the specific treatment lines, OS was estimated from the start date of the treatment line.
PFS was estimated from the index date until progression, death of any cause, or end of follow-up, whichever occurred first. For specific treatment lines, PFS was estimated from the start date of the specific line.
ToT was estimated from initiation of the first treatment after the index until termination of the last (in case of regimens containing two or more compounds), with death as a competing event. For the specific treatment lines, ToT was estimated within the specific line.
Statistical analysis
All statistical analyses were done according to a pre-specified statistical analysis plan
Continuous variables were described by means and ranges and categorical variables by counts and proportions. To test for differences between stratified groups at index, an unpaired t-test was used for continuous variables (age), a chi2-test was used for categorical variables, and Fischer’s exact test was used for categorical variables with small cell counts. OS and PFS were estimated using the Kaplan–Meier method including 95% confidence intervals and the log-rank test was used to test for differences between the stratified groups. ToT was estimated by the cumulative incidence function accounting for death as a competing risk and a Gray K-Sample test was used to test for differences between the stratified groups.
Clinical outcomes (PFS, OS, ToT) were assessed overall, per treatment line and per treatment modality, stratified by the presence of CNS metastases. All tests applied a significance level of 0.05. All statistical analyses were performed using SAS Enterprise Guide v. 7.15 (SAS Institute, Inc, Cary, NC) and RStudio Version 1.2.1335 (RStudio, Inc., Boston, MA).
Approvals
The study is register-based and does not involve contact with patients. By Danish law, no ethical board approval is required for this type of study. The study was approved in the DBCG’s scientific committee for medical oncology. The study was registered and approved by the Capital Regions research overview (P-2020-1124).
Results
Study population
From January 1st, 2014 to December 31st, 2019, 272 women initiated 3rd line or later treatment for HER2-positive mBC following treatment with T-DM1 in the metastatic setting (). Patient characteristics and demographics are given in .
Figure 1. Flow diagram of patient population describing number of patients and reason for exclusion.
Table 1. Baseline characteristics at index stratified by presence of CNS metastases.
The mean age at inclusion was 59 years. Patients with CNS metastases were significantly younger with a mean age of 54 years whereas patients without CNS metastases had a mean age of 62 (p < .0001, ). At index, visceral metastases were present in 227 (83%) patients. 77 (28%) patients had CNS metastases with 11 (4%) patients having CNS metastases as the only site. Of 272 patients 71 (26%) were diagnosed with primary metastatic breast cancer. At index 194 patients had 3 or more metastatic sites. The population with CNS metastases had significantly more metastatic sites (p = .001, ). The median number of treatment lines received at the index was 3 (range 2–11).
Treatment
Prior to inclusion, all patients received T-DM1 in the metastatic setting with 5 (2%) patients receiving T-DM1 in 1st line, 125 (46%) in 2nd line, 52 (19%) in 3rd line, 43 (16%) in 4th line and the remaining 47 (17%) in the 5th to 11th line. Of the 272 included patients, 167 (61%) were treated with pertuzumab and 269 (99%) with trastuzumab. The population with CNS metastases at index were treated more often with other anti-HER2 (p = .04, ), than the population without CNS metastases.
In the line following inclusion 43 (16%) patients received capecitabine combined with trastuzumab, 32 (12%) received a taxane combined with trastuzumab, 26 (10%) received capecitabine combined with lapatinib, 23 (8%) received T-DM1 again due to progression caused by CNS metastases but with continued systemic response to T-DM1 treatment, 21 (8%) received eribulin combined with trastuzumab, 21 (8%) received experimental treatment and 106 (38%) received 36 different treatment combinations (). Across all lines, 159 (58%) patients discontinued treatment due to progression or adverse events, and 77 (28%) patients died while on treatment.
Table 2. Number of patients per treatment modality in line one to six after inclusion.
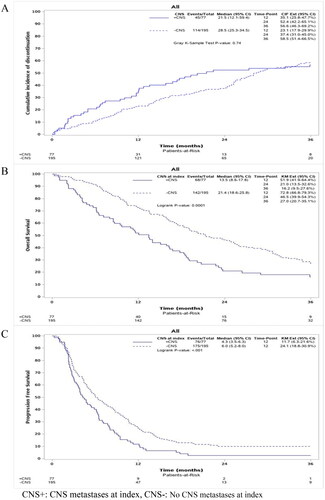
The median time on treatment across all lines and all treatment modalities, with death as a competing risk, was 28.5 months (95% CI, 25.3–34.5) for the group without CNS metastases at index and 21.5 months (95% CI, 12.1–59.4) for the group with CNS metastases at index (). Of the 272 patients included in this study, 159 discontinued their treatment. 77 patients experienced death as a competing event before discontinuing treatment.
Figure 2. (A) Cumulative incidence in % describing time on treatment of patients across all lines stratified by presence of CNS metastases at index. (B) Kaplan–Meier estimates of overall survival (%) according to time since index date, including all patients stratified by presence of CNS metastases at index. (C) Kaplan–Meier curve of progression-free survival (%) according to time since index date, including all patients stratified by presence of CNS metastases at index.
Overall survival and progression-free survival
From inclusion until the end of follow-up, 210 patients died and 251 experienced a progression or death as the first event. Median OS across the entire population was 18.5 months (95% CI, 16.2–21.3). shows that patients with CNS metastases at index had a significantly worse outcome compared to patients without CNS metastases at index, with a median OS of 13.5 months (95% CI, 8.6–17.8) compared to 21.4 months (95% CI 18.6–25.8) (p < .001).
Across the entire population, the median PFS in the 1st line following inclusion was 5.5 months (95% CI, 4.8–6.5). The median PFS was 6.0 months (95% CI, 5.2–8.0) for patients without CNS metastases at index and 4.3 (95% CI, 3.5–6.3) for patients with CNS metastases at index showing a significantly worse outcome for patients with CNS metastases at index (p < .001, ).
Discussion
In this real-world study, we included all registered patients with HER2-positive metastatic breast cancer between 2014 through 2019 who were previously treated with T-DM1 and received 3 or more lines in the metastatic setting. Across the total population, the median PFS was 5.5 months with the median OS being 18.5 months and 28% of the patients had CNS metastases. For patients with CNS metastases at index the median PFS was 4.3 (95% CI, 3.5–6.3) and without 6.0 months (95% CI, 5.2–8.0) with median OS being 13.5 months (95% CI, 8.6–17.8) and 21.4 months (95% CI 18.6–25.8) (p < .001), respectively. In Denmark, the standard treatment of HER2-positive metastatic breast cancer is dual blockade with trastuzumab and pertuzumab accompanied by vinorelbine in 1st line followed by TDM-1 in 2nd line (DBCG guidelines). To our knowledge, no nation-wide real-world data on post-T-DM1 treatment in the metastatic setting has been published.
HER2-positive mBC is, as aforementioned, associated with the development of CNS metastases, none of the standard treatments used during the inclusion period have shown noticeable results in treating CNS metastases. New treatments that can treat CNS disease are needed and several of the new drugs have shown promising results [Citation7].
In our study, the 3rd most used treatment modality in the line subsequent to inclusion was lapatinib combined with chemotherapy. A phase III, randomized study examined lapatinib combined with capecitabine in pretreated HER2-positive mBC patients. The median PFS was 8.4 months for the 163 patients in the group receiving lapatinib combined with capecitabine and 4.1 months for the group receiving capecitabine as monotherapy [Citation10]. Compared to the patient population in our study, that showed a median PFS of 5.5 months, the median PFS was longer, but the patient population differs on several characteristics. In our study, the patient population was heavily pretreated in the metastatic setting and had received a median of three lines before inclusion, whereas the population receiving lapatinib combined with capecitabine in the randomized study had received only trastuzumab as anti-HER2 therapy. Furthermore, only 2% of the patients in the randomized study had CNS metastases compared to 28% in our study.
Despite our study population having a larger percentage of CNS metastases (28%) and being considerably larger, the outcomes remained similar. Another real-world, multicenter study [Citation11] analyzed a population of 325 patients. It showed a median age of 59 years with a median line of post-T-DM1 treatments of four. The median PFS was 6.1 months (95% CI, 5.3–6.7) and the median OS was 23.7 (95% CI, 20.7–27.4). Compared to our study the results are similar without noticeable differences in outcomes. Other studies with similar design showed no real difference in outcome in comparison to our study [Citation12,Citation13].
A large number of trials have been conducted to identify the effect of new drugs, such as trastuzumab-deruxtecan, neratinib, and tucatinib, that might be potential third-line treatment options in the near future. Due to the potential difference in study populations, comparing cross-study/trials should be done with caution, but it is nevertheless important to evaluate both real-world studies and RCTs when approving or assessing the new possible standard of care options for mBC.
Trastuzumab-deruxtecan (T-DXd) was studied in the two-part, open-label, single-arm, multicenter, phase 2 DESTINY-Breast01 study in 2020. The trial included 184 patients previously treated with T-DM1. The median number of lines received prior to inclusion was six and the patients were previously treated with trastuzumab (100%), pertuzumab (65.8%), and other HER2 targeting therapies (54.3%). In our real-life nationwide population, the patients had received a lower number of median lines, (3), prior to inclusion, and only 16% received other HER2 targeting treatment compared to 54.3% in the DESTINY-Breast01 study. Across the entire population in the DESTINY-Breast01 study the median PFS was 16.4 months (95% CI, 12.7–NE) and among the 24 patients with CNS metastases the median PFS was 18.1 months (95% CI 6.7–18.1). At the latest data cutoff (15 January 2021) the median OS was 28.4 months (95% CI, 24.6–37.2) with 91 (49.5%) OS events [Citation8,Citation9]Our study showed a median PFS of 5.5 months (95% CI, 4.8–6.5) across the entire population and 4.3 (95% CI 3.5–6.3) for the patients with CNS metastases, thus the patients in DB01 obtained a longer PFS compared to our study. Worth noting is that T-DXd resulted in a longer PFS compared to our study considering how heavily pretreated the T-DXd population in the DESTINY-Breast01 study was, having received median six prior lines compared to the population in our study with a median of three lines. However only 13% of the patients had CNS metastases compared to 28% in our study, and this may play a role in the difference of outcome as patients with CNS metastases are shown to have a worse outcome in general [Citation3,Citation14]. Of reported adverse events (AE) 99.5% had at least one AE with 57.1% having an AE of grade 3 or higher, with the most common AEs being neutropenia 19.6%, nausea 7.6%, and anemia 8.2%. Worth noting is that 13.6% experienced interstitial lung disease of any grade [Citation8].
Furthermore, the effect of T-DXd was studied in DESTINY-Breast03. The study compared T-DXd and T-DM1 in 524 patients in 2nd line, previously treated with trastuzumab and a taxane. Besides trastuzumab and a taxane, 62.1% had received Pertuzumab before receiving T-DXd, and 60.1% had received pertuzumab in the T-DM1 group. The median PFS by BICR (blinded independent central review) was not reached (95% CI, 18.5–NE) in the T-DXd group and 6.8 (95% CI, 5.6–8.2) months in the T-DM1 group. The hazard ratio for disease progression or death from any cause was 0.28 (95% CI, 0.22–0.37) (p < .001). The confirmed ORR showed a significant difference between the treatment arms with 79.7% ORR (95% CI, 74.3–84.4) in the group receiving T-DXd and 34.2% ORR (95% CI, 28.5–40.3) in the group receiving T-DM1. The drug-related AEs of any grade was 98.1% with T-DXd and 86.6% in T-DM1, with the most common AEs being nausea (72.8%), fatigue (44.7%), and vomiting (44%). The incidence of these AEs was lower in the T-DM1 group (27.6%, 29.5%, and 5.7%) respectively [Citation15]. Given the results of the DESTINY-Breast01 and 03, it is anticipated that TDX-d will be offered as a standard of care in the near future provided the National Medicines Councils are willing to approve the drug.
The NALA study was conducted to examine neratinib combined with capecitabine versus lapatinib combined with capecitabine in patients previously treated with two or more HER2-targeted treatments. The study enrolled 621 patients with 303 receiving neratinib combined with capecitabine, 311 receiving lapatinib combined with capecitabine, and 7 receiving no study drug. The study found that PFS for the group treated with neratinib was significantly improved (HR, 0.76; 95% CI, 0.63–0.93) [Citation16]. Median PFS was 5.6 months (95% CI, 4.9–6.9) and 5.5 months (95% CI, 4.3–5.6)for the neratinib group and lapatinib group respectively but with no significant benefit in OS 24.0 months (95% CI 22.1–25.9) and 22.2 (95% CI 20.4–24.0) respectively.
The HER2CLIMB study examined the effect of tucatinib combined with trastuzumab and capecitabine in patients treated with trastuzumab, pertuzumab, and trastuzumab emtansine. The patient population was stratified according to the presence or absence of CNS metastases. 612 patients were enrolled in the study to receive either tucatinib or a placebo. It showed a benefit with a median PFS of 7.8 months (95% CI, 7.5–9.6) for the tucatinib group and 5.6 months (95% CI, 4.2–7.1) for the placebo group in the entire population. Among the patients with CNS metastases, the study showed a median PFS of 7.6 months (95% CI, 6.2–9.5) for the tucatinib group and 5.4 months (95% CI, 4.1–5.7) for the placebo group. The median OS was 21.9 months (95% CI, 18.3–31.0) for the tucatinib group and 17.4 months (95% CI, 13.6–19.9) for the placebo group. Common AEs in the tucatinib group included diarrhea, palmar–plantar erythrodysesthesia syndrome, nausea, fatigue, and vomiting. Diarrhea (12.9%) and elevated aminotransferase (5.4%) levels of grade 3 or higher were more common in the tucatinib-combination group than in the placebo-combination group [Citation17].
A tyrosine kinase inhibitor was used as an experimental drug in both the NALA and HER2CLIMB studies. In the HER2CLIMB study, 47.5% of the patients had CNS metastases compared to 28% in our study. When comparing our study to the HER2CLIMB study, the patient group in the HER2CLIMB study receiving tucatinib obtained a longer median PFS but no improved median OS. The patient group receiving neratinib in the NALA study obtained both a longer PFS and OS, but compared to the population of our study, only 16.6% had CNS metastases at baseline compared to 28% in our study. Comparing the HER2CLIMB and NALA study to one another it may seem, that the higher percentile of patients with CNS metastases, HER2CLIMB 47.5% and NALA 16.6%, at inclusion plays a negative role in the outcome, with the NALA study both showing improved PFS and OS compared to our study and the HER2CLIMB only showing a longer PFS. The rapidly expanding market for HER2-positive mBC raises a question as to when to use these new drugs and for whom. Furthermore, increasing costs of new drugs might force regulatory instances to consider whether to apply special considerations as to who to treat and when.
The strength of this study is that it is conducted within a nation-wide study cohort, ensuring the outcomes and characteristics are representative for an entire nation’s population and not biased toward the clinical practices of the major hospitals. There are limitations to the study as the retrospective design and as with every retrospective observational study, the data collected relies on the treating physician’s record-keeping as well as the data collector’s precise entry of data. Another limitation is that a large proportion of the patients treated with T-DM1 did not progress and were thus not treated in the next line. This could cause a bias in the analysis of prognosis after T-DM1 treatment.
Conclusion
In this real-world study, patients were treated with a wide variety of anti-cancer agents in 3rd line or later in HER2-positive mBC with modest efficacy. However, patients in this study did not have access to newer therapies like tucatinib and T-DXd. Data from the studies have shown good results and thus improvement in PFS and OS is anticipated with the introduction of these agents. New treatment options are rapidly evolving for HER2-positive breast cancer. However, these treatments are very expensive and may not be offered in countries with limited health care resources. Thus, it is important to know the efficacy and treatment patterns of existing treatments to help ensuring the best treatments and optimal use of health resources.
Supplemental Material
Download MS Word (30.6 KB)Disclosure statement
AD: Unrestricted Research Grant: Danish cancer society. TB: Institutional grants from: Danish Cancer Society, Neye, Fonden, Roche, Novartis, Samsung Bioepis, Pfizer, AstraZeneca, Merck Eisai, Venture Oncology Personal: Invited Speaker: Pfizer, Advisory Board: Merck. MJ: Institutional grants from: Samsung Bioepis, Nanostring Technologies, Venture Oncology. A. Knoop: Institutional grant: Roche, Personal from: Advisory Board: Novartis, Advisory Board: AstraZeneca, Advisory Board: MDS, Advisory Board: Roche, Advisory Board: Pfizer, Advisory Board: Eli Lilly Danmark A/S. K.K. Andersen: Personal: Full or part-time Employment: AstraZeneca. S. Rana: Personal: Full or part-time Employment: Daiichi Sankyo Nordics ApS. All other authors have declared no conflicts of interest.
Data availability statement
Due to Danish law data cannot be made publicly available. The corresponding author can be contacted with inquiries regarding data availability.
Additional information
Funding
References
- Wolff AC, Hammond MEH, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast. J Clin Oncol. 2013;31(31):3997–4013.
- Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019;321(3):288–300.
- Maurer C, Tulpin L, Moreau M, et al. Risk factors for the development of brain metastases in patients with HER2-positive breast cancer. ESMO Open. 2018;3(6):e000440–9.
- Bartsch R, Wenzel C, Altorjai G, et al. Capecitabine and trastuzumab in heavily pretreated metastatic breast cancer. J Clin Oncol. 2007;25(25):3853–3858.
- Blackwell KL, Burstein HJ, Storniolo AM, et al. Randomized study of lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28(7):1124–1130.
- Cardoso F, Paluch-Shimon S, Senkus E, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol. 2020;31(12):1623–1649.
- Cesca MG, Vian L, Cristóvão-Ferreira S, et al. HER2-positive advanced breast cancer treatment in 2020. Cancer Treat Rev. 2020;88:102033.
- Modi S, Saura C, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382(7):610–621.
- Saura Manich C, Modi S, Krop I, et al. 279P trastuzumab deruxtecan (T-DXd) in patients with HER2-positive metastatic breast cancer (MBC): updated survival results from a phase II trial (DESTINY-Breast01). Ann Oncol. 2021;32: S485–S486.
- Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355(26):2733–2743.
- Yokoe T, Kurozumi S, Nozawa K, et al. Clinical benefit of treatment after trastuzumab emtansine for HER2-positive metastatic breast cancer: a real-world multi-centre cohort study in Japan (WJOG12519B). Breast Cancer. 2021;28:581–591.
- Bines J, Wang B, Xu F, et al. Real-world data of triplet combination of trastuzumab, lapatinib, and chemotherapy in HER2-positive metastatic breast cancer: a multicenter retrospective study. Front Oncol. 2020;10:271.
- Nakayama T, Yoshinami T, Yasojima H, et al. Real-world effectiveness of post-trastuzumab emtansine treatment in patients with HER2-positive, unresectable and/or metastatic breast cancer: a retrospective observational study (KBCSG-TR 1917). BMC Cancer. 2021;21(1):795.
- Martin AM, Cagney DN, Catalano PJ, et al. Brain metastases in newly diagnosed breast cancer: a population-based study. JAMA Oncol. 2017;3(8):1069–1077.
- Cortés J, Kim SB, Chung WP, et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J Med. 2022;386(12):1143–1154.
- Saura C, Oliveira M, Feng YH, et al. Neratinib plus capecitabine versus lapatinib plus capecitabine in HER2-positive metastatic breast cancer previously treated with ≥2 HER2-directed regimens: phase III NALA trial. J Clin Oncol. 2020;38(27):3138–3149.
- Murthy RK, Loi S, Okines A, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2020;382(7):597–609.
