Abstract
Background
Oligoprogression (OPD) is defined as a condition where limited progression (1–3 metastases) is observed in patients undergoing systemic cancer treatment. In this study we investigated the impact of stereotactic body radiotherapy (SBRT) in patients with OPD from metastatic lung cancer.
Material and Methods
Data from a cohort of consecutive patients with SBRT treated between June 2015 and August 2021 were collected. All extracranial metastatic sites of OPD from lung cancer were included. Dose regimens consisted of mainly 24 in 2 fractions, 30–51 Gy in 3 fractions, 30–55 Gy in 5 fractions, 52.5 Gy in 7 fractions and 44–56 Gy in 8 fractions. Kaplan–Meier method was used to calculate Overall Survival (OS), Local Control (LC), and Disease-Free Survival (DFS) from the start date of SBRT to the event.
Results
Sixty-three patients, 34 female and 29 males were included. Median age was 75 years (range 25–83). All patients received concurrent systemic treatment before the start of the SBRT: 19 chemotherapy (CT), 26 CT plus immunotherapy (IT) or Tyrosin kinase inhibitors (TKI) and 18 IT/TKI. SBRT was delivered to the lung (n = 29), mediastinal node (n = 9), bone (n = 7), adrenal gland (n = 19), other visceral metastases (1) and other node metastases (n = 4). After a median follow-up of 17 months, median OS was 23 months. LC was 93% at 1 year and 87% at 2 years. DFS was 7 months. According to our results, there was no statistically significant correlation between prognostic factors and OS after SBRT in OPD patients.
Conclusions
Median DFS was 7 months, translating into the continuation of effective systemic treatment as other metastases grow slowly. In patients with oligoprogression disease, SBRT is a valid and efficient treatment that may enable postponing the switch of systemic line.
Background
Lung cancer is one of the most aggressive cancers, representing the main cause of cancer-related death among men and the second leading cause among women worldwide [Citation1]. In a significant number of cases, distant metastases are present at the time of diagnosis [Citation2]. Clinical management of metastatic lung cancer underwent radical changes in the last decade. While systemic therapy remains the mainstay of treatment for metastatic lung cancer, advances in the knowledge of tumor microenvironment and identification of driver mutations in a specific subgroup of patients profoundly modified the therapeutic landscape of lung cancer. These discoveries allowed the development of novel targeted agents such as Epidermal Growth Factors Receptor Tyrosine Kinase Inhibitors (EGFR-TKI), and Immune Checkpoint Inhibitors (ICI) [Citation3–11], resulting in improved rates of median progression-free survival and overall survival in the range between 3.5–13.7 months and 9.3–27.7 months, respectively [Citation12]. Nonetheless, most patients will develop over time disease progression (PD) under treatment due to the onset of drug resistance mechanisms. For instance, prolonged administration of targeted therapy with EGFR inhibitors is associated with the emergence of T790M mutation in EGFR exon 20, which hinders the binding between EGFR and drug [Citation13]. Interestingly, the occurrence of mutations correlated to treatment refractoriness is not ubiquitous but frequently occurs in a limited number of progressive sites that may be amenable to a local treatment in order to restore sensitivity to treatment. This assumption led to the concept of oligoprogressive disease (OPD), a condition where limited progression (1–3 metastases) is observed in polymetastatic patients who achieve stable disease under a systemic treatment line [Citation14–15]. Literature data showed that nearly half of patients receiving EGFR-TKI therapy and approximately 13% of patients receiving ICI experience disease oligo progression [Citation16]. Recently, there is an increasing interest in the possibility to offer local ablative treatments, such as stereotactic body radiotherapy (SBRT), in selected oligometastatic patients [Citation17–19]: three Phase II trials showed that metastasis-directed therapy (MDT) in oligometastatic patients could improve not only progression-free survival (PFS), but also overall survival (OS) [Citation20–Citation22]. This paradigm may also be applied in the case of oligoprogression: local treatment of OPD sites may theoretically enable to postpone systemic therapy switch, in particular in patients undergoing novel targeted or immune therapies. However, scarce data are available on the impact of local ablation on OPD. In this study we investigated the outcome after SBRT in patients with metastatic lung cancer treated with chemo-immunotherapy who were diagnosed with oligoprogression.
Material and methods
This is a retrospective multicentric study. Metastatic lung cancer patients over 18 years old were included following SBRT to metastatic sites (all extracranial sites allowed) in case of oligoprogression. Oligoprogression was defined as the onset of up to 3 extracranial metastases in a patient receiving systemic treatment. Stereotactic treatment was delivered using the Cyberknife radiotherapy system (Accuray Inc, Sunnyvale, CA) or the Elekta Versa HD (Elekta AB, Stockholm, Sweden). Simulation CT was performed in free breathing conditions. Clinical Target Volume (CTV) corresponded to the Gross Tumor Volume (GTV). Motion management was granted according to treatment sites using real-time tumor tracking (with or without fiducials) or immobilization with vacuum mattress and daily cone-beam-CT, resulting respectively in 3 or 5 mm isotropic expansion of the GTV to Planning Target Volume (PTV). To allow for comparison of different dose regimens, the dose was expressed as Biological Effective Dose assuming an α/β = 10 (BED10).
Definition of the endpoints
Local failure (LF) was defined as a disease persistence or relapse in the field of treatment. Local control (LC) for the 69 metastases was calculated from the start of radiation therapy to the date of LF or last follow-up. Overall survival (OS) was evaluated from the start of the radiation therapy until death from any cause or last follow-up. Disease-free survival (DFS) was measured from the start of radiation therapy to the occurrence of new metastases outside the irradiated field and/or local failure considering also local failure Acute and Late Toxicity was evaluated according to Common Terminology Criteria for Adverse Events (CTCAE version 5.0).
Statistical analysis
The Kaplan–Meier method was applied to estimate survival curves for LC, DFS, and OS from the start date of SBRT to the event. A p value ≤0.05 was considered statistically significant. Statistical analysis was performed with IBM SPSS v.21 statistical software. The log-rank test was used to compare categorical and continuous variables on overall survival and local control. Univariate and Mutivariate analyses were performed testing prognostic factors related to SBRT in patients with oligoprogression. Variables analyzed for LC were histologic subtype (adenocarcinoma versus other), systemic treatment schedule (chemotherapy alone, immunotherapy/tyrosin kinase inhibitors (IT/TKI) and combination of chemotherapy and IT/TKI), metastatic site of SBRT (pulmonary versus extrapulmonary) and dose to PTV as expressed in BED10. For OS and DFS, sex, age, gender, Karnofsky Performance Status (KPS), systemic treatment schedule (chemotherapy alone, immunotherapy/tyrosin kinase inhibitors and combination of chemotherapy and IT/TKI), number of lines of systemic therapy, presence of brain metastases, the timing of metastases (metachronous or synchronous) were analyzed.
Results
Patient characteristics
We identified 38 patients treated at Erasmus Medical Center (Rotterdam, The Netherlands) and 25 patients at Careggi University Hospital (Florence, Italy) between May 2015 and August 2021: in total, 63 consecutive patients were included. Patients’ characteristics are reported in . Median age was 75 years (range 25–83): 34 patients were female (54%) and 29 were male (46%). All patients had a diagnosis of primary lung cancer, consisting of Non-Small Cell Lung cancer and Small Cell Lung Cancer in 58 (92%) and 5 (8%) patients respectively. Karnofsky Performance Status (KPS) was 90–100 in 54 patients (86%), and <90 in 9 (14%). Synchronous metastatic disease, defined as a metastatic disease at first diagnosis, was found in 45/63 patients (71%). Concerning the global burden of disease, 25 patients (40%) developed more than 10 metastases during the whole disease course (time interval between primary tumor diagnosis and SBRT): among these, 13 patients had more than 10 metastases located in the lung. Fourteen patients (22%) presented with brain metastases. Seventeen patients (27%) had driver mutations. All patients underwent systemic treatment before the start of radiotherapy, consisting of 19 cytotoxic chemotherapy (CT) alone (30%), 26 CT plus immunotherapy (IT) or plus Tyrosin kinase inhibitors (TKI) (41%), and 18 IT/TKI alone (29%). Oligoprogression occurred after 1 systemic therapy line in 34 patients (54%), after 2 lines in 23 (37%) and after more than 2 lines in 6 (9%). The median follow up was 17 months (range 1–56). Six patients presented with two concurrent oligoprogressive sites that were treated with SBRT, accounting for a total number of 69 metastases. Metastatic locations treated with SBRT are summarized in . Dose regimens consisted of 24 Gy in 2 fractions, 30–51 Gy in 3 fractions, 30-55 Gy in 5 fractions, 52.5 Gy in 7 fractions, 44–56 Gy in 8 fractions, and 30 Gy in 10 fractions, resulting in a median BED10 of 104 Gy (range 39–151).
Table 1. Patient, Tumor, and Treatment Characteristics.
Treatment outcomes
After a median follow-up of 17 months, the median OS was 23 months (95% CI 17–29 months): OS at 12 and 24 months was respectively 85% and 49%, (). Median DFS was 7 months (95% CI 4–9 months): DFS at 12 and 24 months was respectively 31% and 11%, (). Median LC was not reached: LC was 93% at 12 months and 87% at 24 months (). As for prognostic factors, we didn’t find a significant correlation with any tested variable. No variables showed a significant statistical correlation with either OS, DFS, or LC. Only a non-significant trend toward improved LC was shown in pulmonary versus extrapulmonary metastases (100 versus 84%, p = 0.052) (Figure S1). Ongoing treatment was continued until SBRT failure and/or further progression. In we show the tumor response to SBRT in an oligoprogressive patient ().
Figure 1. Treatment Outcomes: Kaplan–Meier plots for A: Overall Survival following SBRT. B: Disease Free Survival following stereotactic body radiation therapy (SBRT). C: Local Control following stereotactic body radiation therapy (SBRT).
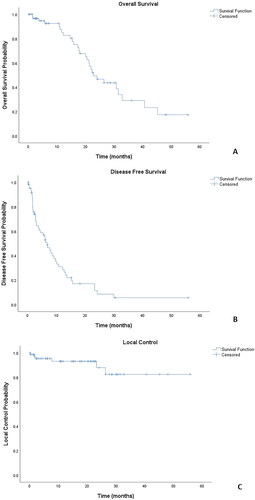
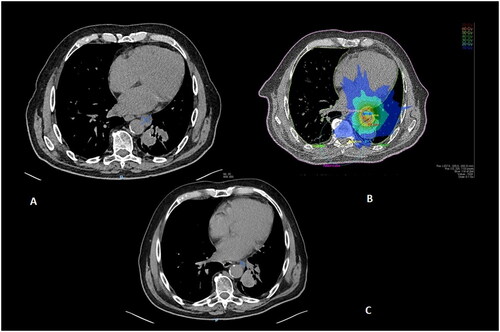
Figure 2. Patient with a clinical history of metastatic NSCLC treated with Nivolumab. A: Oligoprogression consisting of a para-aortic lymphadenopathy detected at follow-up contrast-enhanced chest CT under treatment. B: SBRT delivering 55 Gy in five fractions to the PTV (light blue: 20 Gy; green: 30 Gy; dark green: 40 Gy; yellow: 50 Gy; orange: 60 Gy). C: CT evaluation at six months showing complete response. Patient continued Nivolumab until further polymetastatic progression at 30 months.
Toxicity
No acute grade >2 toxicity was observed. A few cases of acute toxicities were observed: grade 1 pain (n = 1), grade 1–2 dyspnea (n = 5), grade 1 cough (n = 3), grade 1–2 asthenia (n = 5), grade 2 nausea (n = 3) and dysphagia grade 1–2 (n = 3) respectively.
Discussion
In our study, we reported outcomes and adverse events in oligoprogressive lung cancer patients undergoing SBRT for extracranial metastases including lung, bone, nodal and visceral metastasis. We found excellent Local Control and promising Disease Free and Overall Survival rates. Oligoprogression during a systemic treatment may occur in up to 47% of patients [Citation23] and usually requires switch to a further systemic line. This may result in premature discontinuation of effective therapy and consequently, reduce the future availability of alternative systemic treatment options. Use of Metastases Directed Therapy (MDT) such as surgery or Stereotactic Body Radiotherapy in case of limited disease progressions may allow to selective address of the refractory disease sites while maintaining the same treatment, resulting in prolonged disease control. Several trials evidenced the role of MDT in oligometastatic conditions. SABR–COMET, a randomized, phase 2, open-label multicenter trial evidenced an improvement in OS (15 months) in patients with oligometastatic disease (all primary tumors confounded) treated with standard of care plus SBRT versus standard of care only [Citation21]. This was confirmed in the setting of metastatic NSCLC in a phase II trial [Citation20] showing additional benefit on OS of adjunction of SBRT to maintenance chemotherapy. Similar results are found in the recent prospective SINDAS trial (NCT02893332) [Citation24] that evaluated the adjunction of radiotherapy to first-line tyrosine kinase inhibitor (TKI) therapy for EGFR-mutated synchronous oligometastatic NSCLC. The authors concluded that upfront SBRT to sites at diagnosis along with first-line TKI improved both progression-free survival and overall survival significantly compared with TKI alone. However, in the case of oligoprogression the role of SBRT is less clear: there are currently few trials that investigated the role of SBRT in this setting of patients. Moreover, an association of SBRT with oncogenic-driven targeted therapies has been mostly addressed in recent reports, showing variable benefit according to driver mutation. Use of SBRT for OPD in Epithelial Growth Factor Receptor (EGFR) mutated lung cancer has been widely described resulting in reported PFS of 6-18 months [Citation14,Citation25–31] although, the outcome may vary according to tyrosine-kinase inhibiting agent and/or presence of gene variants. In patients receiving the ALK-ROS inhibitor Crizotinib, overall treatment duration in case of OPD raised from 11 to 28 months, suggesting an OS benefit [Citation32]. Focusing on lung cancer patients receiving immune checkpoint inhibitors, a median PFS of 11 months was observed following SBRT to OPD (both intra-and extracranial) in a cohort of 24 patients [Citation33]. In our study we considered only patients with extracranial OPD lung cancer undergoing SBRT after one or multiple systemic therapy lines consisting of immune-based (ICI) or targeted therapies (TKI) and chemotherapy before stereotactic treatment. In our study, the median OS was 23 months. This is comparable with the result from Chan et al. who reported a median OS of 28.2 months in Epidermal Growth Factor Receptor Mutation-positive patients with Oligoprogression, and treated with SBRT [Citation25]: similarly, other studies [Citation26–27] report a median overall survival inferior to 40 months. However, Yu et al. analyzed data from 18 EGFR positive NSCLC patients treated with local therapy (including not only SBRT but also surgical resection and radio-frequency ablation) and concluded higher median OS of 41 months [Citation28]. However, it should be reminded that all these results apply to patients with EGFR-mutated NSCLC and survival results may not be comparable with our cohort including both oncogene-addicted and wild-type disease patients. No clinical or treatment-related factor associated with improved OS was identified. However, the role of unidentified determinants should be thoroughly investigated to identify a subset of patients with expected survival benefit from adjunction of SBRT to the standard of care: in particular assessment of biological predictors linked to treatment resistance such as the onset of primary and secondary mutational variants or loss of expression of target molecules through repeated tissue biopsy or liquid biopsy may be of primary interest. Regarding disease-free survival in our study, we report a median DFS of 7 months, which is consistent with outcomes reported in EGFR-mutated patients. Chan et al. and Friedes et al. reported a median DFS of 7.0 and 7.9 months respectively [Citation25,Citation34]. Rossi et al. [Citation35] reported a trend toward improved time from first to second progression following the adjunction of SBRT to EGFR-directed therapy (6.7 vs 3.1 months, p = 0.06). Weiss et al. analyzed patients treated with the 1st generation EGFR TKI erlotinib and metastatic sites including bone, brain, lymph nodes, and liver. They obtained a time to further progression of 6 months, and the median duration of erlotinib therapy following local treatment was 5.7 months [Citation31]. Concerning patients treated with ICI, the use of SBRT in the event of OPD is scarcely reported. The rationale for the combination of ICI with radiotherapy relies on hypothetical synergistic activity between ionizing radiation and immune-based tumor rejection, as reported by various authors [Citation36–37]. Unfortunately, the phase II Pembro-RT showed only a non-significant trend toward improved DFS in patients receiving SBRT in association with pembrolizumab [Citation38]: however, these results may be partially explained by the use of non-ablative radiotherapy dose schedules. Finally, no reports are available to our knowledge concerning the use of SBRT in patients without driver genomic alterations or immune checkpoint expression. In our study, a median DFS value of 7 months may be relevant with regard to the possibility to postpone the switch systemic line. Interestingly, in our experience, no difference in time to DFS was observed across different treatment groups, showing that SBRT could be applied in both oncogene-addicted disease and in absence of driver mutations. No variables were associated with improved DFS in our experience, as opposed to prior reports: for instance, Friedes and Qiu [Citation34,Citation39] reported improved DFS in patients experiencing OPD more than 6 months after treatment initiation, while Friedes suggested that lower disease burden at presentation (≤3 metastases) may also correlate with longer DFS. As for local control, excellent rates of 93% at 1 year and 87% at 2 year were observed. At univariate and multivariate analysis we did not find prognostic factors with an impact on LC. Interestingly we observed a better local control in patients SBRT treated for a lung metastasis rather than for other visceral metastases. At univariate analysis we obtained a trend toward a statistically significant correlation, p value = 0.052. Similarly to previously published studies, SBRT was well tolerated. We reported essentially grade 1 toxicities and only a few cases of grade 2 for asthenia, dyspnea, dysphagia and nausea. No chronic toxicities were observed, but our median follow-up it was 17 months. There are several limitations of our study. The first one is that the patient population is relatively small. Heterogeneity in systemic treatment was another limit, as well as the different fractionation schedules, but depended on the dose tolerance of different treatment sites. Second, a consensual definition of OPD is lacking. However, no formal definition is available concerning the threshold number of progressive sites in oligoprogressive disease: according to different authors, this may vary between 1 to 5 metastases, with 3 or 5 metastases as the most frequently reported definitions [Citation23]: in order to minimize potential bias linked to multiple organ involvement we opted for a more restrictive threshold. Furthermore, this was a retrospective study performed on patients treated on a long time window, so selection bias such as a change in the Standard of Care (SOC), for instance, a shift from 1st to 3rd generation TKI, over time could not be excluded. Currently, ongoing prospective trials are testing in phase III design the adjunction of SBRT to oncogene-driven (HALT, NCT03256981) wild-type (STOP, NCT02756793) oligoprogressive metastatic lung cancer, while phase II studies are testing its use in PD-L1 expressing tumor (OLCSG 2001, UMIN000041778) or irrespectively of systemic therapy class (SUPPRESS-NSCLC, NCT04405401). In conclusion, a DFS of 7 months was obtained with SBRT. This very low toxic treatment could be a valid option, even if needs to be more investigated, for lung cancer patients with oligoprogressive disease. SBRT in metastatic lung cancer patients with oligoprogression resulted in a DFS of 7 months to further progression. This was associated with a long median overall survival of 23 months, with an excellent local control rate and minimal toxicity. In patients with oligoprogression disease, SBRT is a valid and efficient treatment that may enable to postopone switch of the systemic line. More research is needed to address the gain of the SBRT in patients with oligoprogressive disease across different disease subsets.
Informed consent Statement
Informed consent was obtained from all subjects involved in the study.
Supplemental Material
Download MS Word (11.9 KB)Supplemental Material
Download JPEG Image (72.1 KB)Acknowledgments
None.
Disclosure statement
The authors report there are no competing interests to declare.
Data availability statement
Relevant data supporting the findings of this study are available within the Article or are available from the authors upon reasonable request.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med. 2011; 32(4):605–644.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015; 65(1):5–29.
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092.
- Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–1830.
- Langer CJ, Gadgeel SM, Borghaei H,et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17(11):1497–1508.
- West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(7):924–937.
- Paz-Ares L, Ciuleanu TE, Cobo M, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):198–211.
- Burotto M, Manasanch EE, Wilkerson J, et al. Gefitinib and erlotinib in metastatic non-small cell lung cancer: a meta-analysis of toxicity and efficacy of randomized clinical trials. Oncologist. 2015;20(4):400–410.
- Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet. 2017;390(10089):29–39.
- Lee CK, Brown C, Gralla RJ, et al. Impact of EGFR inhibitor in non-small cell lung cancer on progression-free and overall survival: a meta-analysis. J Natl Cancer Inst. 2013;105(9):595–605.
- Rosell R, Carcereny E, Gervais R,, et al. Erlotinib versus standard chemotherapy as first-line treatment for european patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–246.
- Masters GA, Temin S, Azzoli CG, et al. American society of clinical oncology clinical practice guideline update. J clin oncol. J Clin Oncol. 2015;33(30):3488–3515.
- Lim SM, Syn NL, Cho BC, et al. Acquired resistance to EGFR targeted therapy in non-small cell lung cancer: mechanisms and therapeutic strategies. Cancer Treat Rev. 2018;65:1–10.
- Santarpia M, Altavilla G, Borsellino N, et al. High-dose radiotherapy for oligo-progressive NSCLC receiving EGFR tyrosine kinase inhibitors: real world data. In Vivo. 2020;34(4):2009–2014.
- Kagawa Y, Furuta H, Uemura T, et al. Efficacy of local therapy for oligoprogressive disease after programmed cell death 1 blockade in advanced non-small cell lung cancer. Cancer Sci. 2020;111(12):4442–4452.
- Rheinheimer S, Heussel CP, Mayer P, et al. Oligoprogressive non-small-cell lung cancer under treatment with PD-(L)1 inhibitors. Cancers . 2020;12(4):1046.
- Niibe Y, Hayakawa K. Oligometastases and oligo-recurrence: the new era of cancer therapy. Jpn J Clin Oncol. 2010; 40(2):107–111.
- Cheung P. Stereotactic body radiotherapy for oligoprogressive cancer. BJR. 2016;89(1066):20160251.
- Kim C, Hoang CD, Kesarwala AH, et al. Role of local ablative therapy in patients with oligometastatic and oligoprogressive non-small cell lung cancer. J Thorac Oncol. 2017;12(2):179–193.
- Gomez DR, Tang C, Zhang J, et al. Local consolidative therapy Vs. maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: long-Term results of a multi-institutional, phase II, randomized study. J Clin Oncol. 2019;37(18):1558–1565.
- Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019 May 18;393(10185):2051–2058.
- Iyengar P, Wardak Z, Gerber DE, et al. Consolidative radiotherapy for limited metastatic non-small-cell lung cancer: a phase 2 randomized clinical trial. JAMA Oncol. 2018;4(1):e173501.
- Harada D, Takigawa N. Oligoprogression in non-small cell lung cancer. Cancers 2021;13(22):5823.
- Wang XS, Bai YF, Verma V, et al. Randomized trial of first-line tyrosine kinase inhibitor with or without radiotherapy for synchronous oligometastatic EGFR-mutated NSCLC. J Natl Cancer Inst. 2022;djac015.
- Chan OSH, Lee VHF, Mok TSK, et al. The role of radiotherapy in epidermal growth factor receptor mutation-positive patients with oligoprogression: a matched-cohort analysis. Clin Oncol (R Coll Radiol). 2017;29(9):568–575.
- Conforti F, Catania C, Toffalorio F, et al. EGFR tyrosine kinase inhibitors beyond focal progression obtain a prolonged disease control in patients with advanced adenocarcinoma of the lung. Lung Cancer. 2013;81(3):440–444.
- Weickhardt AJ, Scheier B, Burke JM, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol. 2012;7(12):1807–1814.
- Yu HA, Sima CS, Huang J, et al. Local therapy with continued EGFR tyrosine kinase inhibitor therapy as a treatment strategy in EGFR-mutant advanced lung cancers that have developed acquired resistance to EGFR tyrosine kinase inhibitors. J Thorac Oncol. 2013;8(3):346–351.
- Xu Q, Liu H, Meng S, et al. First-line continual EGFR-TKI plus local ablative therapy demonstrated survival benefit in EGFR-mutant NSCLC patients with oligoprogressive disease. J Cancer. 2019;10(2):522–529.
- Schmid S, Klingbiel D, Aeppli S, et al. Patterns of progression on osimertinib in EGFR T790M positive NSCLC: a Swiss cohort study. Lung Cancer. 2019;130:149–155.
- Weiss J, Kavanagh B, Deal A, et al. Phase II study of stereotactic radiosurgery for the treatment of patients with oligoprogression on erlotinib. Cancer Treat Res Commun. 2019;19:100126.
- Gan GN, Weickhardt AJ, Scheier B, et al. Stereotactic radiation therapy can safely and durably control sites of extra-Central nervous system oligoprogressive disease in anaplastic lymphoma kinase-positive lung cancer patients receiving crizotinib. Int J Radiat Oncol Biol Phys. 2014;88(4):892–898.
- Wang Z, Wei L, Li J, et al. Combing stereotactic body radiotherapy with checkpoint inhibitors after oligoprogression in advanced non-small cell lung cancer. Transl Lung Cancer Res. 2021;10(12):4368–4379.
- Friedes C, Mai N, Fu W, et al. Isolated progression of metastatic lung cancer: clinical outcomes associated with definitive radiotherapy. Cancer. 2020;126(20):4572–4583.
- Rossi S, Finocchiaro G, Noia VD, et al. Survival outcome of tyrosine kinase inhibitors beyond progression in association to radiotherapy in oligoprogressive EGFR-mutant non-small-cell lung cancer. Future Oncol. 2019;15(33):3775–3782.
- Bhalla N, Brooker R, Brada M. Combining immunotherapy and radiotherapy in lung cancer. J Thorac Dis. 2018;10(S13):S1447–S1460.
- Sun X, Gan L, Na A, et al. Combination with stereotactic body radiotherapy offers a promising strategy to overcome resistance to immunotherapy in advanced renal cell cancer. J Oncol. 2019;2019:1483406.
- Theelen WSME, Peulen HMU, Lalezari F, et al. Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol. 2019;5(9):1276–1282.
- Qiu B, Liang Y, Li Q, et al. Local therapy for oligoprogressive disease in patients with advanced stage non-small-cell lung cancer harboring epidermal growth factor receptor mutation. Clin Lung Cancer. 2017;18(6):e369–e373.
