Background
Although oncological treatment of bladder cancer (BC) has recently evolved with the use of immune checkpoint inhibitors (ICI), used as maintenance therapy in metastatic disease [Citation1], and the recent introduction of the anti-Nectin-4 antibody, Enfortumab Vedotin, as a treatment for the relapsed disease after ICI, the survival rates remain modest [Citation2]. Thirty percent of all cancer patients with BC receive chemo- or immunotherapy for muscle-invasive and/or metastatic disease. These treatments in combination with symptoms from the disease result in a vast burden of symptoms. In addition, the BC population is generally described as frail with comorbidities, compromising treatment options and completion [Citation3].
In recent years increasing focus has been paid to health-related quality of life (HRQoL) when introducing new treatments. HRQoL is defined as the subjective perceptions of the positive and negative aspects of cancer patients’ symptoms, including physical, emotional, social, and cognitive functions and disease symptoms and side effects [Citation4]. HRQoL is now frequently a secondary endpoint in large phase III randomized clinical trials investigating the impact on survival of new drugs in BC [Citation5,Citation6] but has until recently been understudied in this patient population [Citation7]. A cross-sectional study from 2021 demonstrates that BC patients have a reduced HRQoL compared to other pelvic cancer patients [Citation8]. Another study on HRQoL among BC patients from 2022 concludes that symptoms, functioning and QoL decrease with more advanced disease [Citation9]. Both studies underline the importance of supportive care interventions, for example in the form of the app developed in the current project.
Several studies have investigated the impact of symptom monitoring by Patient-Reported Outcomes (PROs) during cancer therapy across diagnoses [Citation10–13], and this has recently led to an ESMO guideline on PRO in cancer treatment. The guideline recommends adding PRO to the cancer treatment trajectory to improve parameters such as HRQoL, and symptom burden to gain more knowledge on the real-life use of PRO [Citation14].
The current study is based on the Danish, multicenter, randomized, clinical trial: iBLAD study [Citation15]. The iBLAD study investigated the impact of active PRO compared to usual care for symptom-handling in a BC population and showed that BC patients are willing to participate in PRO research, have high rates of hospitalizations and low rates of treatment completion due to potentially preventable symptoms. With this in mind, it made sense to develop an app that accommodated these patients’ needs for supportive care. Moreover, in a study on the use of ePRO in a fragile and comorbid BC population, the patients had an overall good compliance with ePRO with high questionnaire completion rates (75%) and adherence above 70% throughout six treatment cycles [Citation16]. This is in line with other studies that have shown that cancer patients are willing to complete and adhere well to ePRO interventions such as weekly symptom monitoring [Citation17,Citation18]. In these studies, patient reporting was used actively in the clinical encounter, which may have increased the relevance and the inclination to report for the patients. If the reports are not used actively or if it is unclear if the reports have been viewed by clinicians, the patients may become frustrated [Citation19], which again may lead to poorer adherence. In this study, we expect the intervention to be highly feasible as long as the reports are viewed by the treating clinicians.
The iBLAD study did not show any difference in total HRQoL score or survival between the PRO-intervention and standard care but found a significant difference in emotional function [Citation15].
The aim of this study is to develop and implement a national multimodality app for patients with BC that builds upon knowledge from the iBLAD study. Further, to investigate how the app, containing PRO questions on symptoms and HRQoL, information for health care providers, and peer-to-peer advice, can provide more knowledge on symptoms, HRQoL, and the need for supportive care.
In the iBLAD app study, we will test how symptoms measured using ePRO in a multimodality app are correlated to HRQoL in patients undergoing medical oncological treatment for BC. The hypothesis is that HRQoL in BC is correlated to specific key symptoms during medical oncological treatment as found in the study by Taarnhøj et al. [Citation20], and that specific symptoms occur and influence HRQoL predominantly at specific time points or in time intervals.
Material and methods
Patients
Patients with BC defined as both urothelial tract-, bladder- and urethral cancer initiating first or second-line standard therapy with either chemotherapy or immunotherapy (IO) can participate in the study. Additional inclusion criteria are age ≥ 18 years, performance status ≤ 2, ability to read and understand Danish, and that the patient has given written informed consent.
Exclusion criteria are no access to a smartphone, dementia, cognitive impairment, or psychiatric disease that can compromise informed consent from the patient and/or adherence to the protocol and the monitoring of the trial. All potential participants will be informed about the study by a nurse or physician, who will collect informed consent if the patient wishes to participate.
Design
The iBLAD app study is an investigator-initiated, national, Danish multicenter, exploratory trial connected to the group of BC responsible specialists at the six Danish treatment centers: Copenhagen University Hospital, Herlev Hospital, Næstved Hospital, Odense University Hospital, Aarhus University Hospital, and Aalborg University Hospital. The study is carried out in accordance with the SPIRIT-PRO Protocol Guidance (Supplementary material 1) [Citation21].
Study plan
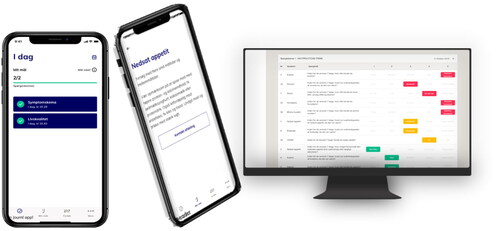
The app is developed by the app provider Journl who is specialized in ePRO apps. The app is available in Danish. The patient will be able to download the app and create a user profile when a clinician has registered the patient in Journl’s system as a patient with BC in oncological treatment. After giving informed consent, the patients will (be helped to) download the app on their private smartphones. The patient will answer the first questionnaires (baseline) in relation to the first treatment. In routine clinincal care, clinicians carry out toxicity registration prior to each cycle of anti-neoplastic treatment, typically every three or four weeks. The weekly patient reporting in the app takes place at home between visits to the hospital.
The first patient was enrolled in December 2022. Patients can use the app as long as treatment is ongoing. Patients can at any time withdraw their consent and stop using the app. The patient will receive daily notifications from the app if there is an unanswered questionnaire to minimize missing data.
Questionnaires
In the app, BC patients will receive electronic questionnaires on symptoms and HRQoL selected by an expert group of clinicians as the most relevant questionnaires for this patient population [Citation21,Citation22]. The questionnaires are the same regardless of the patient’s oncological treatment and cover chemotherapy and ICI. The symptom questionnaire is to be completed weekly and consists of 15 symptoms selected from the PRO-CTCAE library [Citation23]. Each symptom is elicited using between one to three questions on frequency, severity, and interference with daily activities, resulting in 30 questions in total. Moreover, there is a free text field where the patient can add extra symptoms. Weekly reporting is chosen as this is the preferred recall period for PRO-CTCAE questionnaires [Citation24]. Moreover, HRQoL will be measured every month by the EORTC Quality of Life questionnaire (QLQ-C30) and the more disease-specific questionnaire for patients with muscle-invasive BC (QLQ-BML30). The patients are expected to complete the questionnaire themselves.
Some of the features of the app are illustrated in .
Patient feedback and inclusion in the clinical encounter
The patients receive immediate feedback either for supportive care or are advised to contact the hospital as they complete the symptom questionnaire (ePRO-CTCAE) in the app. The type of feedback depends on the patients’ responses and predefined thresholds. Thus, advice is based on an algorithm linked to the severity of the symptoms or side effects that the patients report. If the patients, for example, report a mild symptom, they are given self-management advice directly in the app. If, on the other hand, a severe symptom is reported, the patients are advised to contact the hospital. In the case of a moderate symptom, it depends on the specific symptom whether the patient should self-manage or contact the hospital (see supplementary material for alert algorithms and thresholds for alerts).
The algorithm, including the type of intervention, was developed by an expert group consisting of two physicians/researchers who are experts in BC, its treatment, side effects, and symptom monitoring. The third member of the group was a nurse who had vast experience with symptom monitoring. All three members were experienced PROresearchers.
The symptom and HRQoL reports are sent to the hospital where they can be accessed by clinicians who are trained and prompted to review the scores at patient visits and use them as a dialogue tool in the clinical encounter. The app will enable regular symptom tracking, feedback to patients, and HRQoL reporting to achieve earlier symptom management and improved supportive care. The ePRO questionnaires are added to the standard of care and the symptoms highlighted will be treated in alignment with standard practice.
Peer-to-peer advice
In addition to completing questionnaires in the app, the patients also gain access to several short videos with former BC patients recruited from the Danish BC organization. The rationale is that the patients, through these stories, are provided with a unique chance of mirroring themselves in fellow patients and learning from their experiences, thereby optimizing coping strategies.
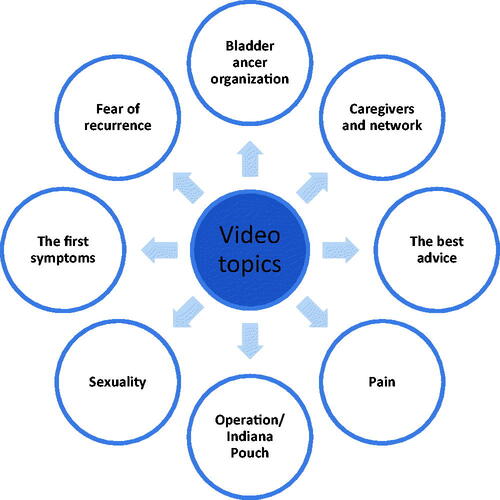
A patient involvement workshop with a panel of patients with BC who had all received anti-neoplastic treatment was held. At the workshop, the six patients took part in deciding what the app should contain in terms of informative and relevant video material on the experiences of BC patients. Fifty-three videos have been produced with five BC patients (three men and two women). In the videos, the patients share peer-to-peer insights on how they experience being BC patients, covering a wide range of topics such as living with cancer, operation, treatment, sexual issues, family relations, rehabilitation, and HRQoL, .
Evaluation
Patients will receive questionnaires on symptoms and HRQoL as long as treatment is ongoing. After 6 - 8 weeks of treatment, the patients will be asked to complete a validated Patient-Reported Experience Measure questionnaire in the clinic to evaluate the acceptability of the app [Citation25]. In addition, individual interviews will be performed with participants in the study from all six centers where usability, patient satisfaction, and adherence is evaluated.
Endpoints
The primary outcome is PRO symptoms’ correlation to HRQoL measured by QLQ-C30 and QLQ-BLM-30 and submodules of these. Additionally, specific PRO symptoms development over time and time-dependency in relation to HRQoL will be investigated. The clinically meaningful difference will be considered a 10-point change in both the total HRQoL-score and the five subdomains (physical, emotional, social, role, and cognitive). The study is non-comparative and analyses will be exploratory. Secondary endpoints are the level of user acceptability, patient satisfaction, and adherence to the app using both quantitative and qualitative methods.
Sample size
No sample size calculation has been performed due to the study’s explorative nature. To secure a broad geographic representation the aim is to include 100 patients with a minimum of 10 patients from each of the six centers. The study will be analyzed when 100 patients have been followed for at least 3 months. Interim analyses will be performed after 30 included patients, primarily to evaluate adherence to the app. In case of poor adherence, selected patients will be contacted and interviewed about their experiences.
Data collection and statistics
Information on age, gender, socio-economic status, time of diagnosis, histology, TNM stage, treatment type, treatment line, and performance status will be noted. A baseline questionnaire on symptoms and HRQoL will be completed. Analyses of data will include descriptive statistics for both changes from baseline and observed scores after 1, 2, 3, 4, 5, and 6 months of participation in the study. In case of missing data, imputation will not take place. Data will be monitored regularly in the beginning every week to ensure adherence. After 12 weeks, we will shift to monthly monitoring if adherence is acceptable. If patients do not comply with the study protocol, they will be contacted by the project manager and/or withdrawn from the study. Eligible patients will be informed about this upon enrollment and that it will have no impact on their treatment.
Conclusion
In this study, a national multimodality app will be developed and implemented, enabling the monitoring of symptoms and HRQoL among bladder BC patients. In addition, self-management advice will be provided to the patients, and the app will serve as a dialogue tool in the clinical encounter. Moreover, patients enrolled in the study will gain access to videos with former BC patients, elucidating relevant topics. Data from 100 BC patients will help better understand the correlation between specific symptoms and HRQoL and symptom development over time. Moreover, user acceptability, patient satisfaction, and adherence will be explored.
Ethics declaration
The iBLAD app study was exempt for review by the Medical Research Ethics Committee. It will follow General Data Protection Regulation and is registered in the Capital Region of Denmark (P-2021-7339). The results of the study will be reported in a scientific journal.
Trial registration: ClinicalTrials.gov Identifier: NCT05710159
Supplemental Material
Download MS Word (49.6 KB)Supplemental Material
Download MS Word (24.5 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that part of the data supporting the findings of this study are available within the article and/or its supplementary materials. The data not included are available from the corresponding author, [LKT], upon reasonable request.
References
- Rhea LP, Mendez-Marti S, Kim D, et al. Role of immunotherapy in bladder cancer. Cancer Treat Res Commun. 2021;26:100296.
- Yu EY, Petrylak DP, O'Donnell PH, et al. Enfortumab vedotin after PD-1 or PD-L1 inhibitors in cisplatin-ineligible patients with advanced urothelial carcinoma (EV‑201): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2021;22(6):872–882.
- Guancial EA, Roussel B, Bergsma DP, et al. Bladder cancer in the elderly patient: challenges and solutions. Clin Interv Aging. 2015;10:939–949.
- EORTC Quality of Life. 2021. https://qol.eortc.org/quality-of-life/
- Bitting RL, Healy P, George DJ, et al. Phase II trial of enzalutamide and androgen deprivation therapy with salvage radiation in men with high-risk prostate-specific antigen recurrent prostate cancer: the STREAM trial. Eur Urol Oncol. 2021;4(6):948–954.
- McGregor B, O'Donnell PH, Balar A, et al. Health-related quality of life of patients with locally advanced or metastatic urothelial cancer treated with enfortumab vedotin after platinum and PD-1/PD-L1 inhibitor therapy: results from cohort 1 of the phase 2 EV-201 clinical trial. Eur Urol. 2022;81(5):515–522.
- Taarnhøj GA, Johansen C, Pappot H. Quality of life in bladder cancer patients receiving medical oncological treatment; a systematic review of the literature. Health Qual Life Outcomes. 2019;17(1):20.
- Catto JWF, Downing A, Mason S, et al. Quality of life After bladder cancer: a cross-sectional survey of patient-reported outcomes. Eur Urol. 2021;79(5):621–632.
- Smith AB, McCabe S, Deal AM, et al. Quality of life and health state utilities in bladder cancer. Bladder Cancer. 2022;8:55–70.
- Di Maio M, Basch E, Bryce J, et al. Patient-reported outcomes in the evaluation of toxicity of anticancer treatments. Nat Rev Clin Oncol. 2016;13(5):319–325.
- Beaver CC, Magnan MA. Managing chemotherapy side effects: achieving reliable and equitable outcomes. Clin J Oncol Nurs. 2016;20(6):589–591.
- Coolbrandt A, Van den Heede K, Vanhove E, et al. Immediate versus delayed self-reporting of symptoms and side effects during chemotherapy: does timing matter? Eur J Oncol Nurs. 2011;15(2):130–136.
- Basch E, Deal AM, Kris MG, et al. Symptom monitoring With Patient-Reported outcomes During routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557–565.
- Di Maio M, Basch E, Denis F, et al. The role of patient-reported outcome measures in the continuum of cancer clinical care: ESMO clinical practice guideline. Ann Oncol. 2022;33(9):878–892.
- Taarnhøj GA, Johansen C, Carus A, et al. 1556MO The iBLAD study: patient-reported outcomes in bladder cancer during oncological treatment: a multicenter national randomized controlled trial. Ann Oncol. 2022;33: s 1259–S1260.
- Taarnhøj GA, Lindberg H, Dohn LH, et al. Electronic reporting of patient-reported outcomes in a fragile and comorbid population during cancer therapy - a feasibility study. Health Qual Life Outcomes. 2020;18(1):225.
- Tolstrup LK, Bastholt L, Dieperink KB, et al. The use of patient-reported outcomes to detect adverse events in metastatic melanoma patients receiving immunotherapy: a randomized controlled pilot trial. J Patient Rep Outcomes. 2020;4(1):88.
- Basch E, Schrag D, Henson S, et al. Effect of electronic symptom monitoring on Patient-Reported outcomes Among patients With metastatic cancer: a randomized clinical trial. JAMA. 2022;327(24):2413–2422.
- Tolstrup LK, Pappot H, Bastholt L, et al. Patient-Reported outcomes During immunotherapy for metastatic melanoma: mixed methods study of patients’ and clinicians’ experiences. J Med Internet Res. 2020;22(4):e14896–e14896.
- Taarnhøj GA, Johansen C, Lindberg H, et al. Patient reported symptoms associated with quality of life during chemo- or immunotherapy for bladder cancer patients with advanced disease. Cancer Med. 2020;9(9):3078–3087.
- Calvert M, King M, Mercieca-Bebber R, et al. SPIRIT-PRO extension explanation and elaboration: guidelines for inclusion of patient-reported outcomes in protocols of clinical trials. BMJ Open. 2021;11(6):e045105.
- Taarnhøj GA, Lindberg H, Johansen C, et al. Patient-reported outcomes item selection for bladder cancer patients in chemo- or immunotherapy. J Patient Rep Outcomes. 2019;3(1):56.
- National Cancer Institute. Patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). 2019. https://healthcaredelivery.cancer.gov/pro-ctcae/
- Mendoza TR, Dueck AC, Bennett AV, et al. Evaluation of different recall periods for the US national cancer institute’s PRO-CTCAE. Clin Trials. 2017;14(3):255–263.
- Tolstrup LK, Pappot H, Zangger G, et al. Danish translation, cultural adaption and initial psychometric evaluation of the patient feedback form. Health Qual Life Outcomes. 2018;16(1):77.