Abstract
Aim
To investigate the pathological complete response (pCR) achieved after neoadjuvant therapy with versus without adding pertuzumab (P) to trastuzumab (H) plus neoadjuvant chemotherapy (NCT) in HER2+ breast cancer (BC) patients in a real-life setting.
Methods
A total of 1528 female HER2+ BC patients who received NCT plus H with or without P were included in this retrospective real-life study. Primary endpoint was pCR rate (ypT0/Tis ypN0). Clinicopathological characteristics, event-free survival (EFS) time, and relapse rates were evaluated with respect to HER2 blockade (NCT-H vs. NCT-HP) and pCR.
Results
Overall, 62.2% of patients received NCT-H and 37.8% received NCT-HP. NCT-HP was associated with a significantly higher pCR rate (66.4 vs. 56.8%, p < 0.001) and lower relapse (4.5 vs. 12.2%, p < 0.001) in comparison to NCT-H. Patients with pCR had a significantly lower relapse (5.6 vs. 14.9%, p < 0.001) and longer EFS time (mean(SE) 111.2(1.9) vs. 93.9(2.7) months, p < 0.001) compared to patients with non-pCR. Patients in the NCT-HP group were more likely to receive docetaxel (75.0 vs. 40.6%, p < 0.001), while those with pCR were more likely to receive paclitaxel (50.2 vs. 40.7%, p < 0.001) and NCT-HP (41.5 vs. 32.1%, p < 0.001). Hormone receptor status and breast conservation rates were similar in NCT-HP vs. NCT-H groups and in patients with vs. without pCR. Invasive ductal carcinoma (OR, 2.669, 95% CI 1.596 to 4.464, p < 0.001), lower histological grade of the tumor (OR, 4.052, 95% CI 2.446 to 6.713, p < 0.001 for grade 2 and OR, 3.496, 95% CI 2.020 to 6.053, p < 0.001 for grade 3), lower T stage (OR, 1.959, 95% CI 1.411 to 2.720, p < 0.001) and paclitaxel (vs. docetaxel, OR, 1.571, 95% CI 1.127 to 2.190, p = 0.008) significantly predicted the pCR.
Conclusions
This real-life study indicates that adding P to NCT-H enables higher pCR than NCT-H in HER2+ BC, while pCR was associated with lower relapse and better EFS time.
Introduction
In patients with human epidermal growth factor receptor-2 (HER2) with early breast cancer (BC), neoadjuvant therapy is used to downstage tumors, increase breast conservation rates, and more importantly assess response to treatment which ultimately provides important prognostic information regarding the risk of recurrence and guides future adjuvant treatment strategies [Citation1,Citation2]. Pathological complete response (pCR), defined as the absence of residual invasive cancer in the breast and axilla (ypT0/is, ypN0) at surgery after neoadjuvant treatment [Citation3,Citation4], is considered a reliable surrogate marker of long-term clinical outcome and survival in HER2+ BC patients [Citation2,Citation4–7].
Accordingly, use of dual HER2 blockade with pertuzumab (P) and trastuzumab (H) plus chemotherapy has become a mainstay of neoadjuvant therapy in HER2-positive early or locally-advanced BC patients, based on the pCR benefit, reducing the risk of recurrence and mortality demonstrated primarily in the clinical trial setting [Citation2,Citation7–12].
However, despite the well-recognized differences between real-life evidence and randomized clinical trials, limited data are available regarding the pCR rate achieved after neoadjuvant dual HER2 blockade (P and H) in combination with chemotherapy in early HER2-positive BC patients in real-life, routine practice [Citation2,Citation6,Citation7,Citation13,Citation14].
This multicenter, nationwide, real-life HER2PATH study aimed to investigate the pCR rate following neoadjuvant therapy with versus without adding P to H plus chemotherapy in HER2+ BC patients and to evaluate clinicopathological characteristics and clinical outcomes in subgroups of HER2 blockade (with vs. without pertuzumab).
Materials and methods
Study population
A total of 1528 female HER2+ BC patients who received neoadjuvant chemotherapy (NCT) plus H with or without P were included in this nationwide retrospective cohort study (ClinicalTrials.gov Identifier: NCT04765124) conducted at 21 tertiary care referral centers in Turkey, between 2015 and 2021. Data collection was completed between April 2021 and December 2021.
HER2 status was determined by immunohistochemical (IHC) staining. Tumors having a score of 3 (+) were considered as HER2-positive. Tumors scoring 2 (+) for HER2 expression were subsequently analyzed by fluorescence in situ hybridization (FISH) and were considered as HER2-positive if HER2 amplification was present in FISH. HER2 FISH positive result was defined as HER2/CEP17 ratio ≥2.0 or average HER2 copy number ≥6.0 signals per cell. Estrogen (ER) and progesterone receptor (PR) nuclear staining ≥ 1% was accepted as ER and/or PR-positive by IHC evaluation according to the ASCO/CAP guidelines. Baseline ER, PR, and HER2 status were based on core biopsies at the diagnosis.
Pertuzumab was started to be used in Turkey after March 2019, at least the first 25 patients who used pertuzumab after March 2019 from 21 participating centers and the 50 most recent patients who did not use pertuzumab and completed NCT were included. Thereafter, patients were divided into two groups based on the type of HER2 blockade including NCT-H (taxane plus trastuzumab, n = 951) and NCT-HP (taxane plus trastuzumab with pertuzumab, n = 577) groups. Female patients aged ≥18 years who had been diagnosed with histologically confirmed HER2+ BC and treated with H plus taxane with or without P as neoadjuvant therapy and underwent breast surgery at the participating centers were included in the study. The study was conducted in accordance with the ethical principles stated in the ‘Declaration of Helsinki’ and approved by the Istanbul Medipol University Non-Interventional Clinical Research Ethics Committee (Date of Approval: 25/05/2021; Reference number/Protocol No: 488/ML42876).
Data collection
Data on diagnosis age, menopausal status, initial ECOG performance status (PS), tumor pathology (histological subtype, TNM stage, histological grade, lymphovascular invasion, and perineural invasion), hormone receptor (HR) status, Ki67, concomitant chemotherapy, surgery, pCR rate and oncological outcome (EFS time and relapse rate) were recorded. The pCR rate (ypT0/Tis ypN0) was the primary endpoint. Demographic, clinicopathological characteristics, clinical outcomes, and pCR rates (yes vs no) were evaluated with respect to HER2 blockade (NCT-H vs. NCT-HP). Multivariate logistic regression analysis was also performed to identify the factors predicting pCR.
Safety data were retrospectively collected from patients charts in all patients who had received at least one dose of the NCT plus H with or without P. Adverse events (AEs) were categorized according to National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) (version 4.0).
Treatment protocols
Patients received dose-dense (dd) doxorubicin and cyclophosphamide (AC) (60/600 mg/m2) every 2 weeks with granulocyte colony-stimulating factor support or AC every 3 weeks for 4 cycles, FEC (fluorouracil 600 mg/m2, epirubicin 90 mg/m2/mg and C 600 mg/m2) or FAC protocol (luorouracil 500 mg/m2, doxorubicin 50 mg/m2/mg and C 500 mg/m2) every 3 weeks for 3–4 cycles, followed by weekly paclitaxel (80 mg/m2) for 12 weeks, or docetaxel (75 mg/m2, escalating, if tolerated, to 100 mg/m2) every 3 weeks for 4 cycles with H (8 mg/kg loading dose followed by 6 mg/kg) and P (840 mg loading dose followed by 420 mg) every 3 weeks from the start of taxane. Treatment protocols were preferred according to the age, comorbidities and ECOG PS of patients.
Statistical analysis
Statistical analysis was done using IBM SPSS Statistics for Windows, version 22.0 (IBM Corp., Armonk, NY). A Pearson Chi-square (χ2) test was used for the comparison of categorical data, while an independent-sample t test or a Mann-Whitney U test was used for the parametric variables. Predictors of pCR were determined via multivariate logistic regression analysis performed with the best multivariate model selection (Bayesian Information Criteria (BIC)). If a variable had a significant effect on the score in univariate, it was included in the multivariate analysis. The type 1 error level was set at 5%. Survival analysis was done via Kaplan-Meier analysis and comparisons were made via the Log-Rank test. Data were expressed as ‘‘mean (standard deviation, SD)’’, median (minimum–maximum), 95% confidence interval (CI), and percentage (%) where appropriate.
Results
Clinicopathological characteristics and oncological outcome in the overall study population
The median age at diagnosis was 47.0 years (ranged, 20 to 88 years) and 56.1% of patients were premenopausal. Most patients had an ECOG PS of 0 (74.5%) and invasive ductal carcinoma (83.3%). Progesterone and estrogen receptor positivity were noted in 46.8% and 57.1% of patients, respectively ().
Table 1. Clinicopathological characteristics and oncological outcome in NCT-H vs. NCT-HP groups.
The type of taxane was paclitaxel in 46.4% of patients and docetaxel in 53.6%. Overall, 951 (62.2%) patients received NCT-H, and 577 (37.8%) patients received NCT-HP. pCR was achieved by 60.4% of patients overall. For patients with no events, median follow-up time was 39 months (range: 1–124). However, the median survival time was not reached, while the mean EFS time was 103.9 months ().
Clinicopathological characteristics and oncological outcome in NCT-HP vs. NCT-H groups
The premenopausal status (60.0 vs. 53.7%, p = 0.019), ECOG PS of 0 (82.5 vs. 69.6%, p < 0.001), invasive ductal carcinoma (95.0 vs. 76.2%, p < 0.001), TX-T0-T1 tumors (45.2 vs. 39.7%, p = 0.037), grade 2–3 tumors (86.3 vs. 77.0%, p < 0.001), Ki-67 > 14 tumors (93.8 vs. 87.9%, p = 0.001) and receiving docetaxel treatment (75.0 vs. 40.6%, p < 0.001) were significantly more common in the NCT-HP group than in the NCT-H group (, ).
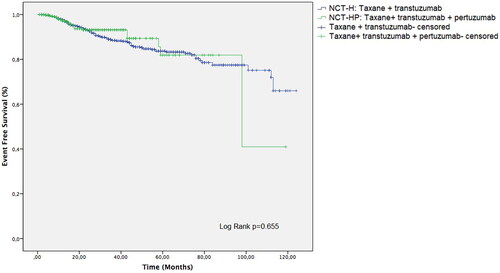
Figure 1. Clinicopathological characteristics, pCR achievement and oncological outcome in NCT-H vs. NCT-HP groups; *p < 0.05 and **p < 0.001 compared to NCT-H group.
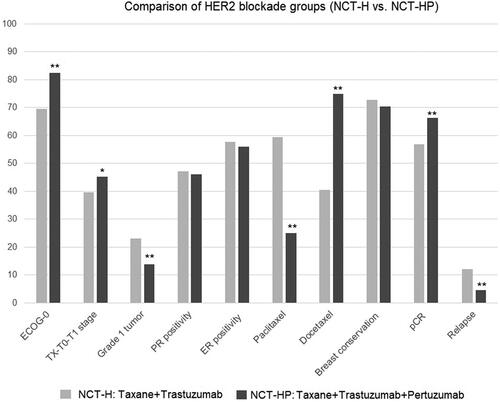
NCT-HP vs. NCT-H treatment was associated with significantly higher rates of pCR (66.4 vs. 56.8%, p < 0.001) and a lower rate of relapse (4.5 vs. 12.2%, p < 0.001) (, Figure (12)).
Clinicopathological characteristics and clinical outcome with respect to pCR rate
In patients with vs. without pCR, the diagnosis age was significantly younger (median: 46.0 vs. 48.0) years, p = 0.040), while having ECOG PS of 0 (76.1 vs. 72.1%, p = 0.042), invasive ductal carcinoma (88.1 vs. 76.0%, p < 0.001), NX-N0 tumors (31.5 vs. 19.3%, p < 0.001), TX-T0-T1 tumors (49.7 vs. 29.8%, p < 0.001), grade 1 tumors (25.8 vs. 9.2%, p < 0.001) and Ki-67 < 14 tumors (11.3 vs. 7.4%, p = 0.034) were significantly more common (, ).
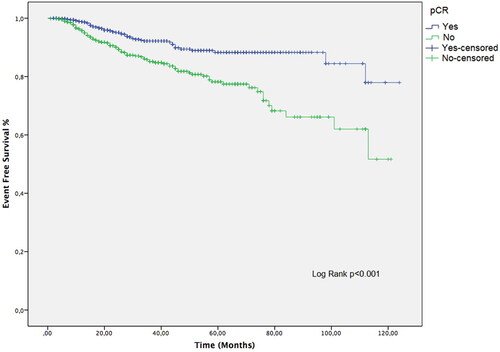
Figure 3. Clinicopathological characteristics, pCR achievement and oncological outcome in patients with vs. without pCR; *p < 0.05 and **p < 0.001 compared to patients without pCR.
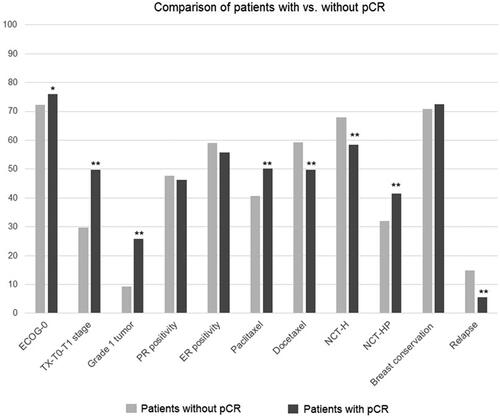
Table 2. Clinicopathological characteristics and oncological outcome with respect to pCR achievement.
Patients with vs. without pCR were more likely to receive paclitaxel (50.2 vs. 40.7%, p < 0.001) or NCT-HP treatment (41.5 vs. 32.1%, p < 0.001), while the presence of pCR was also associated with a significantly lower rate of relapse (5.6 vs. 14.9%, p < 0.001) and longer EFS time (mean(SE, 95% CI) 111.2 (1.9,107.5–114.9) vs. 93.9 (2.7,88.5–99.2) months, p < 0.001; median EFS was not reached in both arms) (, ,]). Patients with non-pCR were continued with adjuvant H in two groups because TDM-1 and P are not available for adjuvant treatment at the time of treatment in Turkey.
Logistic regression analysis for factors predicting achievement of pCR
In univariate logistic regression analysis for pCR, age, Ki-67 status, histological type, histological grade, T stage, lymph node status, the type of taxane and HER2 blockade were found to be significant factors. Thereafter, a multivariate logistic regression analysis was performed in order to further evaluate all of the significant factors for predicting pCR. According to multivariate logistic regression analysis, invasive ductal carcinoma (vs. medullary, OR, 2.669, 95% CI 1.596 to 4.464, p < 0.001), grade 1 tumor vs. grade 2 and 3 (OR, 4.052, 95% CI 2.446 to 6.713, p < 0.001 and OR, 3.496, 95% CI 2.020 to 6.053, p < 0.001, respectively), lower T stage (TX-T0-T1 vs T2-T3-T4, OR, 1.959, 95% CI 1.411 to 2.720, p < 0.001) and paclitaxel vs. docetaxel (OR, 1.571, 95% CI 1.127 to 2.190, p = 0.008) were found to significantly predict the likelihood of achieving pCR ().
Table 3. Logistic regression analysis for factors predicting achievement of pCR.
Safety
In NCT-H and NCT-HP groups, diarrhea (16.0% and 27.0%, respectively), upper respiratory tract infection (12.7% and 14.9%, respectively) and headache (11.7% and 14.0%, respectively) were the most common adverse events. Most common grade ≥3 adverse events in NCT-H and NCT-HP groups included neutropenia (1.7% and 0.7%, respectively) and peripheral edema (1.2% and 0.5%, respectively). Only 3 patients (two in the T arm and 1 in the P arm) had mild decreased left ventricular ejection fraction (LVEF). However, no significant (≥10% points from baseline to <50%) or serious (symptomatic LV systolic dysfunction) declines in LVEF were detected. There was no toxicity leading to death ().
Table 4. Safety data.
Discussion
This real-life study of HER2+ BC patients in the neoadjuvant setting revealed that adding P to NCT-H was associated with more favorable clinical outcomes than NCT-H alone such as higher pCR and 2-year EFS rates and a lower risk of relapse. Patients with pCR had higher 2-year EFS rates, longer EFS time, and a lower risk of relapse; they also had better ECOG PS (ECOG PS 0), lower T stage (TX-0-1), grade 1 tumors, and less use of docetaxel (vs paclitaxel) compared to the patient group without pCR.
The pCR (ypT0/Tis ypN0) rate of 66.4% in our NCT-HP group is consistent with favorable pCR rates reported with the use of neoadjuvant H and P therapy and various chemotherapy backbone in previous real-life studies [Citation2,Citation6,Citation13–15]. In the real-life multicenter NEOPETRA study, among 243 patients with HER2+ early BC, NAC with P and H was reported to be associated with a total pCR (tpCR, ypT0/is ypN0) rate of 66.4% [Citation2]. In a retrospective study with 19 HER2+ non-metastatic BC patients with neoadjuvant dual anti-HER2 blockade and taxane, treatment was reported to reveal high pCR rates of 68% [Citation13]. In another retrospective study in patients with HER2+ BC who underwent neoadjuvant treatment with doxorubicin and cyclophosphamide followed by paclitaxel, H, and P (THP) in the neoadjuvant setting, the tpCR (ypT0/is ypN0) rate was reported to be 72% [Citation15].
Notably, the pCR rates achieved after neoadjuvant therapy in our NCT-HP group are also comparable or even higher than the pCR rates (range, 39 to 68%) reported across the clinical trials with use of neoadjuvant dual HER2 in combination with different chemotherapy backbones in early-stage HER2+ BC patients [Citation9,Citation16–19]. In the NEOSPHERE trial of 417 patients, P, H, and docetaxel treatment was associated with significantly higher a pCR (ypT0/is) rate than trastuzumab and docetaxel treatment (45.8% vs. 29.0%) [Citation9]. In the BERENICE and GeparSepto trials with neoadjuvant H, P, and standard anthracycline/taxane-based chemotherapy, the pCR (ypT0/is ypN0) rates were 61.8% and 66.2%, respectively [Citation16,Citation18], while the TRAIN-2 trial revealed the tpCR rate (ypT0/is ypN0) of 67% and 68% with neoadjuvant dual HER2 blockade and chemotherapy with or without anthracyclines, respectively [Citation20].
Considering the survival outcome, the 2-year EFS rates (90.4% overall, 95% in the NCT-HP group) is consisitent with the data from pivotal studies with dual HER2 blockade in combination with chemotherapy, which revealed high survival rates across the treatment arms such as a 3-year progression-free survival (PFS) rate of 87–89% in the TRYPHAENA trial [Citation21], a 3-year EFS rate of 94.2% in the KRISTINE trial [Citation22] and a 5-year PFS rate of 86% in the NEOSPHERE trial [Citation10]. Also, in a real-life retrospective study of 447 patients with early or locally-advanced HER2+ BC who underwent neoadjuvant docetaxel/carboplatin/H/P (TCHP) therapy, the pCR rate was reported to be 64%, while the 3-year EFS rate was 90.6% [Citation6]. In another real-life study with 78 HER2+ early BC patients, the NCT with H and P was reported to be associated with a pCR (ypT0/is/N0) rate of 46.3%, along with favorable 12-month PFS (99%), 24-month PFS (95%) and 2-year overall survival (OS, 86%) estimates [Citation14].
In our study, the use of NCT-HP was associated with significantly higher pCR rates, while achievement of pCR was associated with significant survival benefits in terms of higher 2-year EFS rates as well as a lower risk of relapse. These findings indicate a further increase in survival with the addition of P to NCT-H treatment, which exceeds the increase in pCR rates (from 19% to 38%) as well as the survival benefit (hazard ratio (HR) 0.64 for EFS and HR 0.66 for OS) reported in HER2+ BC patients treated with NCT-H alone [Citation23,Citation24].
Our findings support that the real-life data on dual HER2 blockade plus chemotherapy among HER2+ BC patients reveal much higher pCR rates than that was reported in randomized clinical trials [Citation4,Citation25], and targeting the HER2 pathway with dual blockade (H and P) can further increase the pCR rate compared with T alone [Citation2,Citation6,Citation8,Citation17].
Indeed, a strong correlation between pCR and prognosis after neoadjuvant therapy has been consistently reported with improved EFS in patients who achieve a pCR after neoadjuvant therapy than those with residual disease, particularly in patients with HER2+ and triple-negative BC [Citation3,Citation7,Citation26,Citation27]. In the NEOSPHERE trial, a greater 5-year PFS was reported in locally-advanced or early-stage HER2+ BC patients with vs. without pCR (85 vs. 76%) after neoadjuvant P and T [Citation10]. A failure to achieve pCR was also reported to be associated with significantly poorer disease-free survival (DFS) than those with pCR (HR, 1.98) in the retrospective observational JBCRG-C03 study among HER2+ primary BC patients treated with NCT plus H alone [Citation27].
Previous studies revealed that HR negativity, low clinical stage, and postmenopausal status were favorable to pCR, while the clinical stage and pCR also had a significant impact on EFS [Citation2,Citation6,Citation14]. However, in our study HR status had no significant impact on the pCR rates. Likewise, the Collaborative Trials in Neoadjuvant Breast Cancer (CTNeoBC) pooled analysis indicated that pCR following NCT combined with H was associated with an improved long-term survival outcome (HR 0.39 for EFS and 0.34 for OS) in the HER2+ subgroup, regardless of the HR status [Citation3]. While HR negativity was associated with increased pCR rates, it is also associated with shorter EFS and PFS. On the other hand, tumor burden (T size and nodal involvement) was significantly associated with survival regardless of pCR status [Citation6]. In our study, lower T stage (TX-T0-T1 vs. T2-T3-T4), lower tumor grade (grade 1 vs. grade 2 and 3), histological type (invasive ductal vs. medullary carcinoma), taxane type (paclitaxel vs. docetaxel) significantly predicted pCR, while the age, menopausal status, EGOG PS, HR status, Ki-67, lymph node positivity, surgery type, anthracycline treatment or addition of P to NCT-H were not among the significant determinants of pCR. The fact that the use of paclitaxel is associated with more pCR rate may possibly be related to its more tolerability and less toxicity than docetaxel in daily practice. Our findings were compatible with BERENICE trial [Citation18]. This seems notable given that patients with initially high clinical stage BC are considered to be at risk of disease recurrence even though pCR had been achieved after neoadjuvant therapy [Citation6]. Analysis of data from five clinical trials conducted by the German Breast Group indicated that baseline clinical nodal stage (HR 1.70) and tumor stage (HR 1.61) were independently correlated with DFS after surgery in patients with pCR [Citation7,Citation28,Citation29].
Our findings also support the consideration of NCT-HP to be a well-tolerated therapy in HER2+ early BC patients associated with a favorable safety profile and no serious cardiac toxicity or treatment-related deaths in the real-life setting, consistent with safety findings reported in clinical trials such as the TRYPHAEA and BERENICE trials [Citation2,Citation7,Citation14,Citation17,Citation18]. In addition, a pooled analysis performed by Swain et al. evaluated the risk of recurrence and death in patients with HER2+ early BC who achieve a pCR after different types of neoadjuvant anti-HER2 therapy [Citation30]. They showed that patients with pCR had longer EFS than those with residual disease. Moreover, patients treated with HP in both the neoadjuvant and adjuvant settings had the lowest risk of breast cancer recurrence. They recommend that some patients who had a pCR still had a risk of relapse; therefore standard of care should be given and further efforts should be made to define prognostic factors for relapse [Citation30].
The major strength of this study is the inclusion of a nationwide database on 1528 HER2+ BC from 21 centers in Turkey, offering large-scale, retrospective real-life data on the effectiveness of dual HER2 blockade plus chemotherapy with the use of a standardized method for pCR assessment. However, certain limitations must be acknowledged. First, the data is retrospective and limited to the therapeutic options available for use in early HER2+ BC within the study period (such as adjuvant TDM-1 and P). Because TDM-1 and P are not available for adjuvant treatment at the time of treatment in Turkey, patients with non-pCR were continued with adjuvant H in two groups. The fact that our study is such a retrospective cohort study can be considered as a kind of selection bias and perhaps influenced our results. Second, a relatively shorter follow-up time is another limitation since a reliable survival outcome analysis needs a longer follow-up interval. This may also have affected our results.
Conclusions
In conclusion, this real-life study indicates the association of neoadjuvant dual HER2 blockade with H and P plus chemotherapy with the achievement of pCR in a high proportion of HER2+ BC patients, with comparable or even higher pCR rates than reported in clinical trials. Having better invasive ductal histology, lower T stage (vs. T2-T3-T4), grade 1 tumors (vs. grade 2 and 3), and taxane type (paclitaxel vs. docetaxel) predicted the achievement of pCR, while patients with vs. without pCR had higher 2-year EFS rates and a lower risk of relapse. Our findings support the efficacy and safety of dual HER2 blockade with P and H plus chemotherapy for neoadjuvant treatment of HER2+ BC patients, which seems to further increase the pCR rate and enables a lower rate of relapse compared with H plus chemotherapy.
Ethical approval
Local Ethics Committee of Istanbul Medipol University approved the study. Further enquiries can be directed to the corresponding author.
Author contributions
All authors contributed to the study conception and design. Material preparation and analysis were performed by Ahmet Bilici. Omer Fatih Olmez, Muhammet al.i Kaplan, Berna Oksuzoglu, Ahmet Sezer,and Nuri Karadurmus. Erdem Cubukcu, Mehmet al.i Nahit Sendur, Sercan Aksoy, Dilek Erdem, Gul Basaran, Burcu Cakar, Abdallah TM Shbair, Cagatay Arslan, Ahmet Taner Sumbul, Sema Sezgin Goksu, Ibrahim Karadag, Irfan Cicin, Mahmut Gumus, Fatih Selcukbiricik, Hakan Harputluoglu, and Umut Demirci contributed to data collection and substantial input to the conception and acquisition of the work. The first draft of the manuscript was written by Ahmet Bilici and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosure statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability statement
This paper was presented in a poster session at the 2022 ASCO Annual Meeting which was held on June 3–7, 2022, in Chicago, IL, and the abstract was published in the Journal of Clinical Oncology, Volume 40, Issue 16_suppl (01 June 2022) e12610-e12610, 02 June 2022. DOI: 10.1200/JCO.2022.40.16_suppl.e12610.
Additional information
Funding
References
- Chen AM, Meric-Bernstam F, Hunt KK, et al. Breast conservation after neoadjuvant chemotherapy: the MD Anderson cancer center experience. J Clin Oncol. 2004;22(12):2303–2312.
- González-Santiago S, Saura C, Ciruelos E, et al. Real-world effectiveness of dual HER2 blockade with pertuzumab and trastuzumab for neoadjuvant treatment of HER2-positive early breast cancer (the NEOPETRA study). Breast Cancer Res Treat. 2020;184(2):469–479.
- Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–172.
- Prowell TM, Pazdur R. Pathological complete response and accelerated drug approval in early breast cancer. N Engl J Med. 2012;366(26):2438–2441.
- Symmans WF, Wei C, Gould R, et al. Long-term prognostic risk after neoadjuvant chemotherapy associated with residual cancer burden and breast cancer subtype. J Clin Oncol. 2017;35(10):1049–1060.
- Kim JY, Nam SJ, Lee JE, et al. Real world evidence of neoadjuvant docetaxel/carboplatin/trastuzumab/pertuzumab (TCHP) in patients with HER2-positive early or locally advanced breast cancer: a single-institutional clinical experience. Cancer Res Treat. 2022;54(4):1091–1098.
- Takada M, Toi M. Neoadjuvant treatment for HER2-positive breast cancer. Chin Clin Oncol. 2020;9(3):32.
- Kreutzfeldt J, Rozeboom B, Dey N, et al. The trastuzumab era: current and upcoming targeted HER2+ breast cancer therapies. Am J Cancer Res. 2020;10:1045–1067.
- Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(1):25–32.
- Gianni L, Pienkowski T, Im YH, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016;17(6):791–800.
- Cardoso F, Kyriakides S, Ohno S, et al. Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow up dagger. Ann Oncol. 2019;30(8):1194–1220.
- Denduluri N, Somerfield MR, Chavez-MacGregor M, et al. Selection of optimal adjuvant chemotherapy and targeted therapy for early breast cancer: ASCO guideline update. J Clin Oncol. 2021;39(6):685–693.
- Choi JDW, Hughes TMD, Marx G, et al. Pathological outcomes of HER2-positive non-metastatic breast cancer patients treated with neoadjuvant dual anti-HER2 therapy and taxane: an Australian experience. Asia Pac J Clin Oncol. 2020;16(3):103–107.
- Hall BJ, Bhojwani AA, Wong H, et al. Neoadjuvant trastuzumab and pertuzumab for early HER2-Positive breast cancer: a real world experience. Breast J. 2022;2022:7146172.
- Singh JC, Mamtani A, Barrio A, et al. Pathologic complete response with neoadjuvant doxorubicin and cyclophosphamide followed by paclitaxel with trastuzumab and pertuzumab in patients with HER2-Positive early stage breast cancer: a single center experience. Oncologist. 2017;22(2):139–143. 13943.
- Loibl S, Jackisch C, Schneeweiss A, et al. Dual HER2-blockade with pertuzumab and trastuzumab in HER2-positive early breast cancer: a subanalysis of data from the randomized phase III GeparSepto trial. Ann Oncol. 2017;28(3):497–504.
- Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol. 2013;24(9):2278–2284.
- Swain SM, Ewer MS, Viale G, et al. Pertuzumab, trastuzumab, and standard anthracycline- and taxane-based chemotherapy for the neoadjuvant treatment of patients with HER2-positive localized breast cancer (BERENICE): a phase II, open-label, multicenter, multinational cardiac safety study. Ann Oncol. 2018;29(3):646–653.
- Shao Z, Pang D, Yang H, et al. Efficacy, safety, and tolerability of pertuzumab, trastuzumab, and docetaxel for patients with early or locally advanced ERBB2-Positive breast cancer in Asia: the PEONY phase 3 randomized clinical trial. JAMA Oncol. 2020;6(3):e193692.
- van Ramshorst MS, van der Voort A, van Werkhoven ED, et al. Neoadjuvant chemotherapy with or without anthracyclines in the presence of dual HER2 blockade for HER2-positive breast cancer (TRAIN-2): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19(12):1630–1640. ED
- Schneeweiss A, Chia S, Hickish T, et al. Long-term efficacy analysis of the randomised, phase II TRYPHAENA cardiac safety study: evaluating pertuzumab and trastuzumab plus standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer. Eur J Cancer. 2018;89:27–35.
- Hurvitz SA, Martin M, Jung KH, et al. Neoadjuvant trastuzumab emtansine and pertuzumab in human epidermal growth factor receptor 2-positive breast cancer: three-year outcomes from the phase III KRISTINE study. J Clin Oncol. 2019;37(25):2206–2216.
- Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol. 2014;15(6):640–647.
- Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375(9712):377–384.
- Fasching PA, Hartkopf AD, Gass P, et al. Efficacy of neoadjuvant pertuzumab in addition to chemotherapy and trastuzumab in routine clinical treatment of patients with primary breast cancer: a multicentric analysis. Breast Cancer Res Treat. 2019;173(2):319–328.
- von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30(15):1796–1804.
- Takada M, Ishiguro H, Nagai S, et al. Survival of HER2-positive primary breast cancer patients treated by neoadjuvant chemotherapy plus trastuzumab: a multicenter retrospective observational study (JBCRG-C03 study). Breast Cancer Res Treat. 2014;145(1):143–153.
- Huober J, Schneeweiss A, Blohmer JU, et al. Abstract P2-08-01: factors predicting relapse in early breast cancer patients with a pathological complete response after neoadjuvant therapy – results of a pooled analysis based on the GBG meta-database. Cancer Res. 2019;79(4_Supplement):P2-08-01–P2-08-01.
- Asselain B, Barlow W, Bartlett J, et al.; Early breast cancer trialists’ collaborative group (EBCTCG). Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018;19:27–39.
- Swain SM, Macharia H, Cortes J, et al. Event-free survival in patients with early HER2-positive breast cancer with a pathological complete response after HER2-Targeted therapy: a pooled analysis. Cancers (Basel). 2022;14(20):5051.