?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Shoulder and arm dysfunction such as reduced range of motion (ROM) and seroma formation, are common complications following axillary lymph node dissection (ALND). There are conflicting results on the effect of early postoperative exercise on the risk of seroma. This study aims to present incidence of symptomatic seroma formation in a large, population-based cohort, and assesses whether early shoulder mobilization, and other common patient and treatment-related factors are predictors of seroma.
Methods
This observational cohort study at the Surgical clinic at Lund University Hospital in Sweden, included 217 consecutive patients who underwent ALND due to breast cancer, cutaneous malignant melanoma (CMM), or carcinoma of unknown primary. A shoulder exercise program was introduced on the first postoperative day and data were collected at routine follow-up 4–6 weeks postsurgery. Main outcome was the strength of the associations between postsurgery exercise and seroma incidence based on logistic regression analyses, supported by data on seroma volume and number of aspirations.
Results
Two hundred patients completed the study. The overall seroma incidence was 67.5% and the odds of seroma were lower for patients practicing ROM exercise two times/day versus 0–1 time/day (OR 0.42, 95% CI 0.18–0.96, p = .038). ROM exercise greater than two times/day did not increase the volume, neither did the arm cycling exercise. ALND combined with mastectomy and CMM surgery were associated with larger seroma volumes (1116 ± 1068ml, p = .006) and (1318 ± 920 ml, p < .001), respectively, compared to the breast conserving surgery (537 ± 478ml) while neoadjuvant chemotherapy showed no influence. The effect of age, patients ≥60 years compared to younger, or BMI ≥ 30.0 were weaker (p = .08).
Conclusions
Extensive surgical treatments for breast cancer and malignant melanoma produces more seroma, and higher age and obesity may also influence the risk. ROM exercises twice daily predict a lower incidence of seroma following ALND, and more frequent shoulder exercise do not increase the volumes.
Introduction
Breast cancer is the most common cancer among women in Sweden, with more than 7500 cases in 2021 [Citation1], and for cutaneous malignant melanoma (CMM), about 4500 invasive melanomas are diagnosed every year [Citation2]. For both breast cancer and CMM, surgery is the primary treatment and staging by axillary lymph node dissection (ALND) is indicated for clinically manifest nodal metastasis [Citation3]. ALND is also still considered standard practice in patients with high burden sentinel lymph node-positive disease.
Following mastectomy and/or ALND, serous fluid accumulates under the skin flaps or in the axillary dead space. The serous fluid, seroma, formed in the axilla seems to be a peripheral fluid similar to lymph [Citation4]. Seroma formation is the most common complication following breast cancer surgery and/or ALND. Excessive accumulation will stretch the skin giving a feeling of tightness and causes discomfort. If the seroma becomes symptomatic, patients may require repeated aspirations of the fluid.
Although not all patients develop symptoms of fluid build-up, seroma is increasingly being considered as a side effect of surgery rather than a complication [Citation4] and the reported incidence varies in different studies between 15% and 90% [Citation5,Citation6]. Although various factors have been suggested to correlate with seroma formation [Citation4,Citation7,Citation8], evidence is still scarce to identify patients who would likely develop seroma. Some studies have reported a higher incidence by more extensive surgery [Citation6–8] and a high body mass index (BMI) has been suggested to associate with increased seroma formation [Citation4,Citation6,Citation8].
Several studies have shown that obesity, as well as a sedentary lifestyle, increase the risk for developing breast cancer [Citation9,Citation10]. Physical activity reduces the risk of cancer recurrence [Citation11] and may also contribute to an adequate body weight. Therefore, it is important to initiate physical activity as soon as possible after surgery.
Shoulder and arm dysfunction such as reduced range of motion (ROM) of the shoulder, muscle weakness, and pain are common complications after ALND [Citation12–14] and may delay the start of postoperative physical activity. Therefore, these complications call for the development and implementation of preventive strategies. In Sweden, early postoperative shoulder mobilization is recommended, and that the patients should return to normal daily activities, including regular exercises, as soon as possible. However, it has been suggested that early shoulder mobilization may cause increased seroma formation [Citation4,Citation6,Citation15]. Although immobilization of the shoulder may prevent seroma [Citation15], the return to normal physical activity could be delayed. Also, other studies have demonstrated no difference in seroma formation when comparing early (within 1–2 days postoperatively) or late (5–7 days postoperatively) initiation of shoulder mobilization [Citation4,Citation8,Citation15,Citation16,Citation17].
Heterogeneity of surgical techniques and relatively small patient groups with different clinical outcomes measured have resulted in conflicting results on the incidence of seroma formation and the effect of early postoperative exercise on the risk of seroma. These contradictory findings call for more rigor research in this area with larger sample size [Citation17].
The aims of this study are to (i) determine incidence of symptomatic seroma formation in a large, population-based, and consecutive patient cohort, and (ii) assess whether early shoulder mobilization as well as other common patient and treatment-related factors are predictors of symptomatic seroma formation.
Materials and methods
Setting and patient population
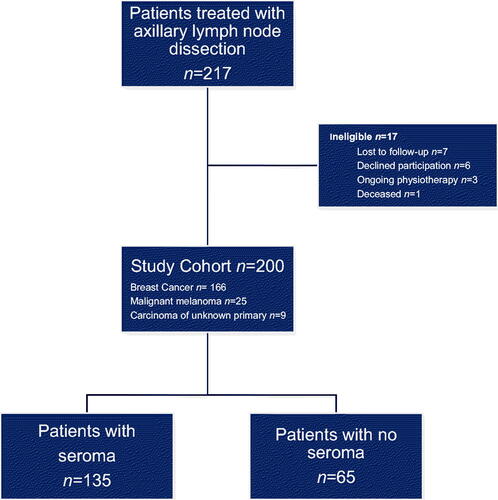
This observational cohort study includes 217 consecutive patients who underwent ALND due to breast cancer, CMM, and carcinoma of unknown primary, at the Department of Surgery, Skåne University Hospital. All patients had axillary drainage following a standard protocol and all were examined 4–6 weeks after surgery according to a routine physiotherapeutic follow-up program. Seventeen patients dropped out and data from 200 patients were available (). One hundred sixty-six (83%) patients were diagnosed with breast cancer, 25 (12.5%) with CMM, and 9 (4.5%) with carcinoma of unknown primary.
Ethical approval
The Regional Ethical Board, Lund University, approved the study, IRB 2017/544. Data were collected within a routine physiotherapeutic follow-up program from October 2014 to December 2016.
Procedure
Definition of symptomatic seroma formation
Seroma formation was defined as excessive accumulation of fluid requiring at least one aspiration due to patient-reported discomfort, documented in the medical record.
Exercise intervention
Before being discharged from the hospital, an implemented routine postoperative exercise program was introduced to all patients by a physiotherapist to improve shoulder ROM, circulation, and to prevent loss of muscle strength. The program started on the first postoperative day and was continued at least until follow-up 4–6 weeks after surgery, when the patient met the same physiotherapist.
The exercise program consisted of five exercises, where exercises 1–4 should be carried out in supine position, two sessions daily. Exercises 1–3 should be repeated five times and exercise 4 [Citation18] should be performed once during each session. Exercise 5 [Citation19] could be performed in any comfortable position several times a day.
Instruction for exercise (Steps 1–5):
Deep breathing exercise with your hands placed on your breast.
Activate your scapular muscles by pressing your arms close to the body and slowly push your arms down along the body.
Elevate both arms over the head as far upwards as possible.
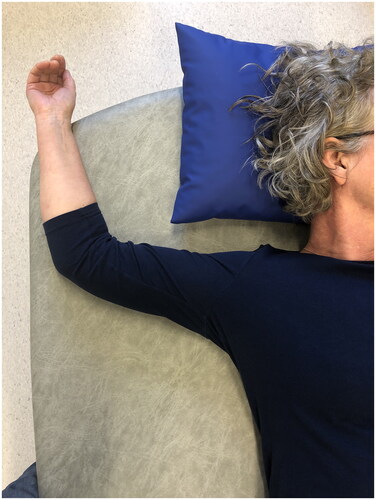
Place your arm on the operated side at a right angle to your body with your elbow bent. Stay in this position relaxing for 2–3 minutes until the feeling of tightness eases ().
Move your shoulder and arm in 10–15 cycling movements, and at the same time clench and open the fist in a pumping action.
The patients were also encouraged to use the arm in daily activities as normally as possible.
Data collection
Clinical data and information on given treatment related to seroma formation were retrieved from medical records: age at diagnosis, BMI (underweight < 18.5, normal weight 18.5–24.9, pre-obesity 25.0–29.9, obesity ≥ 30.0 kg/m2) [Citation20], type of surgery for breast cancer (mastectomy or breast conserving), laterality of ALND, levels of axillary clearance (Ι–ΙΙΙ), neoadjuvant chemotherapy, seroma aspiration frequency, seroma volume, and time from surgery to last aspiration.
Data related to exercise were collected from a standardized physical therapy follow-up protocol after ALND, where the patient responded to a study questionnaire regarding arm dominance, frequency of ROM exercises (0–1 time/day, 2 times/day, or >2 times/day), exercise 5 (arm cycling, 0–4 times/day or 5–10 times/day), spontaneous elevation of the arm ≥ 90° more than five times a day in daily activities, patient's experience of increase seroma formation related to arm activity in general (yes/no).
Statistical analysis
Statistical computations were carried out using IBM SPSS Statistics version 27.
Descriptive statistics of the variables including rates for binary variables and mean ± SD for continuous variables. Comparison between groups with or without seroma formation were performed with Chi Square test for categorical variables and T-test (two-tailed) for continuous variables.
Logistic regression analysis was performed, and odds ratios were used to quantify the strength of the associations between predictors and outcome variable (dependent variable: seroma/no seroma). No imputation was used for missing data. A significance level of p < .05 was chosen.
Results
Patient characteristics
For the overall cohort, the mean age was 61 ± 14.9 (range 25–92) years and mean BMI was 27.0 ± 4.8 (range 15.6–42.3) kg/m2. Mean time to follow-up visit was 4.5 ± 0.9 (range 3–9) weeks and mean time to the last aspiration in the seroma group was 4.0 ± 3.0 (range 1–21) weeks. Frequency of seroma and total seroma volume (mean ± SD) in different categories of cancer treatments, physical exercise intensity, body mass index (BMI), and age, by axillary node dissection, are presented in .
Table 1. Frequency of seroma and total seroma volume (mean ± SD) in different categories of cancer treatments, physical exercise intensity, Body Mass Index (BMI), and age, by axillary node dissection (n = 200).
Number of seroma aspirations
The total number of seroma aspirations per patient is shown in . Just over a quarter (26.7%) of the patients with seroma needed one aspiration only, but the majority of the patients (68.9%) needed 1–3 aspirations and most cases (84.5%) resolved within a month after 1–4 aspirations. However, three patients needed 12, 15, and 16 aspirations, respectively, without there being an obvious reason for these higher frequency of repeated aspirations. Patients requiring 1–7 aspirations had on average seroma volume of 336 ± 222 ml (range 20–1504 ml) at each aspiration ().
Table 2. Frequency of aspiration presented in groups with number of patients, after ALND, (n = 135), with seroma split into each frequency group, and total number of patients in each frequency group with the mean ± SD volume and min–max values.
Seroma volume
Volume related to cancer treatment, BMI, and age
One hundred thirty-five patients, revealing an incidence of 67.5% had seroma formation, 110 (81.5%) with breast cancer, and 19 (14.1%) with CMM. Total aspirated seroma volume per patient was 1048 ± 1039 (range 55–6825) ml.
Significantly more seroma volume was found in patients treated with mastectomy (1116 ± 1068 ml, p = .006) and CMM surgery (1318 ± 920 ml, p < .001) compared to those treated with breast conserving surgery (537 ± 478 ml) (). No differences in seroma volume were found related to the use of neoadjuvant chemotherapy. In the BMI categories, there was a tendency (p = .08) toward larger seroma volume in the obesity group (1212 ± 923 ml) compared to the under/normal weight group (848 ± 888 ml). Concerning age, there was a tendency (p = .08) toward significantly larger seroma formation in the older (≥ 60 years) age group.
Volume related to exercise program
A tendency (p = .068) to less seroma was found for ROM exercise (step 1–4) two times/day (918 ± 839 ml) compared to zero to one times/day (1271 ± 1231 ml). ROM exercise greater than two times/day did not increase the volume; neither did the arm cycling exercise. Side of performed ALND (dominant versus nondominant arm) did not have a significant influence on seroma volume. Evaluation of the arm position during daily activity demonstrated that 80.5% of the patients elevated the arm above the shoulder level (> 90°) more than five times a day. Most of the patients with seroma (72.6%) did not observe any increased amount of fluid build-up following arm activity, while 7.4% of the patients reported increased seroma after ipsilateral arm movement. Twenty percent of the patients stated that they did not know or were not able to comment on seroma formation in relation to arm activity.
Risk for seroma formation
In comparison with ROM exercise zero to one time/day, incidence of symptomatic seroma formation was significantly lower in those practicing ROM exercise two times/day (OR 0.42, 95% CI 0.18–0.96, p = .038) (). Evidence for the discriminatory effect of complete ALND (level I–III) on seroma formation was weak, OR 7.17, (p = .091) and there were no other clinical or treatment variables that was found significantly associated with the incidence of symptomatic seroma formation in univariable logistic regression analysis ().
Table 3. Variables analyzed of group with seroma (n = 135, % of total group) and with no seroma (n = 65, % of total group), and univariate odds ratio (95%CI).
Discussion
The present study demonstrated that twice-a-day exercise, starting on the first postoperative day, was associated with less seroma formation compared to no exercise at all or only once a day. This result is not in line with Shamley et al. [Citation21] who, in their systematic review found support for delayed arm exercise program. However, the interventions were undefined in most of the included studies. In contrast, we have in the present study carefully defined both interventions and frequency of exercise, and the results indicate that a certain level of exercise may facilitate reabsorption of seroma.
When the lymph vessels have closed by themselves and started to develop new vessels [Citation22,Citation23] they will enable removal of the seroma from the operation area. It is well-known that exercises, particularly muscle contractions, can increase the flow, both in veins and lymph vessels [Citation24,Citation25]. Therefore, early start of exercise, twice daily, may be important to support the flow in regenerated vessels as well as in remaining vessels that are forced to transport a larger load of fluid than before the surgery, demanding a higher flow rate. Lymph vessels are also on their own, able to increase flow capacity by increased frequency of lymphangion pumping contractions, also supported by muscle contractions [Citation24].
When patients started the ROM-exercise program on the first postoperative day, they were encouraged to use their arm in daily activities as normally as possible, and the majority were active with the exercise program and in their daily activities. In daily activities, the activity level is usually higher in the dominant than in the nondominant arm, but no differences in amount of seroma were found between the arms, implying that the assumed higher activity in the dominant arm is safe. Further, most patients with seroma (72.6%) did not notice any increase of seroma formation in association with arm activity. Also, there was no difference in seroma volume between low exercise frequency (0–1/day) compared to higher (two or more/day) implying that this group did not reduce their exercise frequency due to larger seroma formation but for other unknown reasons. In addition, the early exercise program is not only beneficial to the patient concerning seroma but may also improve ROM as well as blood and lymph flow.
By complete level I–III ALND, larger damage to the tissue and vessels can be expected and, though not significant (p = .091) the OR for seroma by ALND including level III was very high (7.17). Also, the mean amount of seroma clearly differed between the groups (995 ml in level II/1475 ml in level III). The nonsignificant result is most likely due to a small number of patients and could be expected to be significant if the level III group had been larger. In this context it should also be noted that three patients required repeated aspirations for persisted postoperative seroma (>12 aspirations). All three underwent more extensive surgical treatment with level III ALND or modified radical mastectomy. It has been suggested that the type of surgery is a predicting factor for seroma formation in breast cancer patients. Hashemi et al. [Citation7] demonstrated that modified radical mastectomy was associated with 2.5 times higher risk of postoperative seroma formation. In line with our discussion, SLNB is associated with significantly less seroma formation than conventional ALND due to lesser damage to the lymph and blood vessels [Citation6,Citation8].
In contrast to several studies reporting that high BMI can increase the seroma formation [Citation4,Citation6,Citation8,Citation15,Citation16,Citation26], we did not find any increased risk. However, the difference of seroma formation was large between the under/normal weight (mean 848 ± 888 ml) and obese (mean 1212 ± 923 ml) patients, with a weak association (p = .080). Similar results, with weak association, were found for age, with OR (1.68) (p = .082) in comparison with the younger age group (20–59 years, mean 866 ± 1084 ml) and the older age group (60– 99 years, mean 1180 ± 1018 ml). This is in accordance with a previous study by ten Wolde et al. [Citation27] displaying similar results. It is likely that the wound healing of elderly is delayed. In the present study, some patients were older than 90 years, and even for 60-plus patients there is a risk for less frequent closure of chronic wounds [Citation28]. Neoadjuvant chemotherapy was found to have no significant risk for or influence on seroma formation (), which is in line with several other studies [Citation5,Citation6,Citation7].
The incidence of seroma following ALND varies in different studies between 15% and 90% [Citation5,Citation6]. The overall incidence of seroma formation in the present study was 67.5% revealing that it is a very common side effect after ALND. Eighty-four percent of seromas were found to resolve after one to four aspirations and after about 4 weeks the seroma resolved in most patients. One reason why the incidence of seroma varies so much among studies may be due to different definitions of when seroma aspiration is necessary, which may depend on the patient’s tolerance before they experience that the seroma is uncomfortable. Also, if the time between aspirations is a bit longer there may be some natural reabsorption by the body.
Study strengths and limitations
A strength of this study is that patient sample is rather large (n = 200), making relevant stratification into smaller groups possible, such as for BMI, age, and level of ALND. The data were collected in a clinical environment in a routine physical therapy follow-up using a standardized protocol, which may facilitate implementation in a clinical setting.
A weakness of this study is that there were no standardized criteria when it was time to aspirate the seroma except for when the condition got uncomfortable to the patient. The definition of seroma depended on both the clinical experience of the nurse and the patient's level of tolerance. However, the nurses (n = 4) have many years of experience and could be expected to evaluate the patient's discomfort in a similar way and also being aware of the common adverse effects of frequent aspirations. Also, data were collected retrospectively and could thus present a slightly lower degree of evidence compared to a prospective study. Nevertheless, there was no biased patient selection as all eligible patients were consecutively included. Although limitations of subgroup analysis due to inadequate power do exist, to the best of our knowledge, this is the largest study assessing the risk of seroma with special focus on exercise.
Conclusions
Seroma formation is a common side effect after ALND, however, most cases resolve within a month after a maximum of four aspirations. ROM exercises twice daily predict a lower incidence of seroma formation, and more frequent shoulder exercise do not increase seroma volume. Extensive surgical treatments for malignant melanoma and breast cancer, including ALND at level I–III produces more seroma, compared to less extensive surgery. Higher age and obesity may also influence the risk of increased seroma formation.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data that supports the findings of this study are available on request from the corresponding author, (KJ).
References
- The Swedish National Board of Health and Welfare, Cancer Statistics. Sweden: Socialstyrelsen; 2021 [cited 2022 Oct 13]. Available from: https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/statistik/2021-12-7700.pdf
- Swedish National Guidelines for Malignant Melanoma. Sweden: Regionalt Cancercentrum i samverkan; 2022 [cited 2022 Oct 13]. Available from: https://cancercentrum.se/globalassets/cancerdiagnoser/hud/vardprogram/nationellt-vardprogram-malignt-melanom.pdf
- Faries MB, Thompson JF, Cochran AJ, et al. Completion dissection or observation for sentinel-node metastasis in melanoma. N Engl J Med. 2017;376(23):2211–2222.
- Sitaram PS, Hemanthi R. Seroma formation in cancer breast surgery. JEMDS. 2015;04(10):1681–1688.
- Agrawal A, Ayantunde AA, Cheung KL. Concepts of seroma formation and prevention in breast cancer surgery. ANZ J Surg. 2006; 76(12):1088–1095.
- van Bemmel AJ, van de Velde CJ, Schmitz RF, et al. Prevention of seroma formation after axillary dissection in breast cancer: a systemic review. Eur J Surg Oncol. 2011; 37(10):829–835.
- Hashemi E, Kaviani A, Najafi M, et al. Seroma formation after surgery for breast cancer. World J Surg Oncol. 2004; 2:44.
- Kuroi K, Shimozuma K, Taguchi T, et al. Evidence-based risk factors for seroma formation in breast surgery. Jpn J Clin Oncol. 2006; 36(4):197–206.
- Engin A. Obesity-associated breast cancer: analysis of risk factors. Adv Exp Med Biol. 2017;960:571–606.
- Wisse A, Tryggvadottir H, Simonsson M, et al. Increasing preoperative body size in breast cancer patients between 2002 and 2016: implication for prognosis. Cancer Causes Control. 2018; 29(7):643–656.
- Friedenreich CM, Stone CR, Cheung WY, et al. Physical activity and mortality in cancer survivors: a systematic review and meta-analysis. JNCI Cancer Spectr. 2020; 4(1):pkz080.
- Johansson K, Ingvar C, Albertsson M, et al. Arm lymphoedema, shoulder mobility and muscle strength after breast cancer treatment – a prospective 2-year study. Adv Physiother. 2001;3:55–66.
- Hayes SC, Johansson K, Stout N, et al. Upper-body morbidity following breast cancer: incidence and evidence for evaluation, prevention and management within a prospective surveillance model of care. Cancer. 2012; 118(8 Suppl):2237–2249.
- Kootstra JJ, Dijkstra PU, Rietman H, et al. A longitudinal study of shoulder and arm morbidity in breast cancer survivors 7 years after sentinel lymph node biopsy or axillary lymph node dissection. Breast Cancer Res Treat. 2013; 139(1):125–134.
- Srivastava V, Basu S, Shukla VK. Seroma formation after breast cancer surgery: what we have learned in the last two decades. J Breast Cancer. 2012; 15(4):373–380.
- Shamley DR, Barker K, Simonite V, et al. Delayed versus immediate exercises following surgery for breast cancer: a systematic review. Breast Cancer Res Treat. 2005; 90(3):263–271.
- Petito EL, Esteves MT, Elias S, et al. The influence of the initiation of an exercise program on seroma formation and dehiscence following breast cancer surgery. J Clin Nurs. 2014; 23(21-22):3087–3094.
- McNeely ML, Campbell K, Ospina M, et al. Exercise interventions for upper-limb dysfunction due to breast cancer treatment. Cochrane Database Syst Rev. 2010;(6):CD005211.
- Lauridsen MC, Tørsleff KR, Husted H, et al. Physiotherapy treatment of late symptoms following surgical treatment of breast cancer. Breast. 2000; 9(1):45–51.
- Lane K, Worsley D, McKenzie D. Lymphoscintigraphy to evaluate the effects of upper body dynamic exercise and handgrip exercise on radiopharmaceutical clearance from hands of healthy females. Lymphat Res Biol. 2005;3(1):16–24.
- World Health Organization (WHO). Nutrition - body mass index. Europe, WHO [Internet].cited 2022 Oct 13 Available from: https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi?source=post.
- Suami H, Scaglioni MF, Dixon KA, et al. Interaction between vascularized lymph node transfer and recipient lymphatics after lymph node dissection-a pilot study in a canine model. J Surg Res. 2016; 204(2):418–427.
- Johansson K, Chong H, Ciornei C-D, et al. Axillary web syndrome: evidence for lymphatic origin with thrombosis. Lymphat Res Biol. 2020; 18(4):329–332.
- Olszewski WL, Engeset A, Jaeger PM, et al. Flow and composition of leg lymph in normal men during venous stasis, muscular activity and local hyperthermia. Acta Physiol Scand. 1977;99(2):149–155.
- Havas E, Parviainen T, Vuorela J, et al. Lymph flow dynamics in exercising human skeletal muscle as detected by scintography. J Physiol. 1997;504(1):233–239.
- Kottayasamy Seenivasagam R, Gupta V, Singh G. Prevention of seroma formation after axillary dissection – a comparative randomized clinical trial of three methods. Breast J. 2013; 19(5):478–484.
- ten Wolde B, van den Wildenberg FJ, Keemers-Gels ME, et al. Quilting prevents seroma formation following breast cancer surgery: closing the dead space by quilting prevents seroma following axillary lymph node dissection and mastectomy. Ann Surg Oncol. 2014; 21(3):802–807.
- Sgonc R, Gruber J. Age-related aspects of cutaneous wound healing: a mini- review. Gerontology. 2013;59(2):159–164.