Abstract
Background
We primarily aimed to determine whether the presence of enlarged cardiophrenic lymph nodes (CPLNs), visualized by computed tomography (CT), and CA-125 can be used to assess diaphragmatic carcinomatosis and residual disease (RD) in advanced ovarian cancer (AOC) patients treated with upfront surgery. The secondary aim was to determine the prognostic role of CT-CPLNs in overall survival (OS).
Material and methods
A single-center, retrospective, population-based study was conducted of patients who underwent surgery for AOC from January 1, 2014-December 31, 2018. Suspicious CT-CPLNs were defined as having a short axis ≥5 mm. The median survival and rate of survival were calculated with the Kaplan–Meier method using multivariate Cox regression analyses, including comparisons of complete cytoreductive surgery (CCS; defined as the complete removal of all intra-abdominal tumor) versus noncomplete cytoreductive surgery (non-CCS) and analyses related to CT-CPLN status and preoperative CA-125 values.
Results
We included 208 patients. CT-CPLNs correlated with both diaphragmatic carcinomatosis (OR 3.59, 95% CI 1.81–7.16, p < 0.01) and RD (OR 2.54, 95% CI 1.38–4.6, p = 0.003). When CCS was achieved, no differences in survival between patients with suspicious or nonsuspicious CT-CPLNs were found. The relationships between CA-125 ≥ 500 U/ml and diaphragmatic carcinomatosis (OR 3.51, 95% CI 1.86–6.64, p < 0.01) and RD (OR 2.41, 95% CI 1.33–4.38, p = 0.004) were positive. All data were adjusted for age and ECOG performance status. Survival analyses were also adjusted for RD.
Conclusion
Enlarged CPLNs on CT scans and CA-125 levels correlate with diaphragmatic carcinomatosis and RD at the end of the surgery. The strongest prognostic factor for OS remains CCS, regardless of the CT-CPLN status.
Background
Epithelial ovarian cancer, comprising ovarian, fallopian, and peritoneal cancer, is the leading cause of death from gynecologic malignancies in developed countries [Citation1]. More than two-thirds of cases present at advanced stages (III and IV), defined by the FIGO (Fédération Internationale de Gynécologie et d‘Obstétrique) as disease spread outside of the pelvis [Citation2].
The lack of macroscopic residual disease (RD; defined as any visible intra-abdominal tumor) after a debulking surgery has been demonstrated to be the most important independent prognostic factor in advanced ovarian cancer (AOC) [Citation3]. The standard of care for AOC is primary debulking surgery (PDS) with the intention of complete resection of all macroscopic disease followed by six courses of platinum-based and taxane-based therapy [Citation4]. An alternative approach is neoadjuvant chemotherapy (NACT) followed by interval debulking surgery (IDS), which has shown similar progression-free survival (PFS) and overall survival in two different studies, but this finding is currently being reinvestigated in the Trial on Radical Upfront Surgical Therapy (TRUST), an international open, randomized, controlled multicenter trial [Citation5]. Correctly assessing the extent of disease and the feasibility of a successful PDS and finding tools that assist us in predicting it are of the utmost importance.
Despite being the standard method, the preoperative diagnosis of peritoneal and diaphragmatic carcinomatosis by means of CT scanning poses a challenge, especially in the absence of ascites or lymph nodes larger than 1 centimeter [Citation6]. There is a wide range of results when evaluating the sensitivity of CT scans to diagnose peritoneal carcinomatosis. Sensitivity can vary from 28% to 93% per patient according to previous publications on colorectal cancer, while Luger et al. found a sensitivity of 64% in detecting carcinomatosis of the upper abdomen in ovarian cancer [Citation7,Citation8] Therefore, it would be interesting to identify surrogate markers of peritoneal carcinomatosis given that there is usually an association between upper abdominal disease and a high peritoneal carcinomatosis index (PCI) that could indicate a larger challenge in achieving macroscopically complete tumor resection [Citation8,Citation9].
Some studies have reported that the presence of enlarged cardiophrenic lymph nodes (CPLNs) is associated with peritoneal involvement and worse overall survival and is a possible predictive parameter for nonoptimal debulking surgery [Citation8,Citation10–12]. However, surgically removing CPLNs does not seem to improve survival in patients who have undergone surgery and have zero residual disease, despite their negative effect on survival [Citation13]. This study aims to investigate the role of CPLNs in residual disease and survival in the upfront setting. In a recent study, the addition of CA-125 as a parameter in a combined score with CPLNs improved the predictive value of achieving complete intra-abdominal tumor resection [Citation8]. The purpose of the present study was to add to the literature in terms of the role of CPLNs and CA-125 in foreseeing the extent of diaphragmatic carcinomatosis, the capability of achieving complete macroscopic cytoreduction, and its relationship with overall survival.
Material and methods
The study is a retrospective, population-based analysis of all the patients with primary epithelial ovarian cancer, stage III and IV, treated in a single tertiary center between 2014 and 2018. All patients underwent upfront surgery at the Gynecology Department of Skane University Hospital, Lund. The inclusion criteria were as follows [Citation1]: advanced ovarian cancer [Citation2], upfront cytoreductive surgery [Citation3]; preoperative thorax and abdomen CT images available; and [Citation4] follow-up data until March 2022. The exclusion criteria were as follows [Citation1]: interval debulking surgery [Citation2]; palliative treatment; and [Citation3] incomplete follow-up data. All patient data were handled according to the World Medical Association’s Declaration of Helsinki 2013 and in compliance with Swedish national law. The Swedish Ethical Review Authority approved this study (apl.no. 2019/00450).
The clinical data, including age, ECOG performance status, and CA-125 level, were registered. Because of the skewed distribution, CA-125 data were both logarithmized and dichotomized into two groups: <500 U/ml and ≥500 U/ml.
All eligible patients underwent CT scanning of the thorax and abdomen in the supine position and the majority with intravenous and oral contrast. By convention, all digital CT images were reformatted in the coronal and sagittal planes (all planes 5 mm slice thickness). The data were analyzed by two radiologists, and according to the European Society of Urogenital Radiology (ESUR), all CPLNs with a short-axis ≥5 mm were defined as suspicious (CT-CPLNs) [Citation14].
All surgical debulking procedures were performed in the Gynecology Department of Skane University Hospital, which is a tertiary center for cancer care in Sweden. The medical history, clinical examination and imaging data of all patients were preliminarily discussed in a multidisciplinary manner, and a therapy plan was designed. Surgeries included pelvic procedures, medium abdomen procedures and upper abdomen procedures. Depending on the surgical result, the patients were categorized into a group with complete cytoreductive surgery (CCS), including all patients with zero RD at the end of the surgery, or in a group with RD of any size (non-CCS). Intraoperative data, including carcinomatosis on the diaphragmatic surfaces, were registered.
Overall survival was defined as the time interval from the date of surgery to the date of death or last follow-up. To analyze the possible differences in survival in relation to CT-CPLN status, the patients were matched in pairs: CT-CPLN <5 mm vs. ≥5 mm and CCS vs. non-CCS. The association between CT-CPLNs ≥5 mm and clinicopathological parameters was analyzed using Mann–Whitney U or chi-square tests. The median survival and the rate of survival were calculated using the Kaplan–Meier method and multivariate Cox regression analyses for the CCS and non-CCS groups and the CT-CPLN status, respectively. A log-rank test was used to compare the statistical significance of differences between the Kaplan–Meier curves. A p value and a confidence interval were generated to calculate the statistical significance. A p value of <0.05 was considered significant. All statistical analyses were performed using the statistical software IBM SPSS Statistics for Windows, version 26.0 (released in 2019 by IBM Corp., Armonk, NY).
Results
Descriptive data
Overall, 208 patients with AOC were identified as eligible for this study. The median age in the whole group was 68.3 years, and the median follow-up time was 42.6 months. The FIGO distribution was as follows: twelve patients (5.7%) had FIGO stage IIIA, 15 (7.2%) had FIGO stage IIIB, 133 (63.6%) had FIGO stage IIIC, 23 (11%) had FIGO stage IVA and 25 (12%) were classified as FIGO stage IVB.
In 132 patients (63.5%), complete cytoreduction was achieved, while 76 patients (36.5%) had the residual disease at the end of the surgery (28.4% residual disease <10 mm and 8.2% residual disease ≥10 mm). In 5 patients, the data on CT-CPLN status were unclear, and these patients were excluded from further analyses.
In 88 patients (43.3%), radiologically enlarged CPLNs with short axes ≥5 mm were registered. Patients with enlarged CT-CPLNs were associated with a higher CA-125 level at baseline (mean value 1165 ± 1401.6 U/ml) compared with those with unsuspicious CT-CPLNs (660 ± 11021 U/ml; p = 0.000). An association was found between enlarged CT-CPLNs and patient age at diagnosis (66.6 vs. 67.3 years, p < 0.01). Intraoperative diaphragmatic carcinomatosis was registered in 140/203 (67.4%) of the patients. The patient characteristics are presented in .
Table 1. Patient characteristics.
The role of CPLNs in preoperative evaluation of diaphragmatic carcinomatosis and the risk of residual disease
Logistic regression analyses showed a positive relationship between CT-CPLNs and diaphragmatic carcinomatosis (OR 3.66, 95% CI 1.85–7.24, p < 0.01) and between CT-CPLNs and residual disease (OR 2.37 95% CI 1.32–4.25, p < 0.004). After adjusting for age and ECOG status, the positive relationship between CT-CPLNs and diaphragmatic carcinomatosis and residual disease was maintained (OR 3.59, 95% CI 1.81–7.16, p < 0.01 and OR 2.54, 95% CI 1.38–4.6, p = 0.003, respectively).
The role of CA-125 in the preoperative evaluation of diaphragmatic carcinomatosis and the risk of residual disease
The mean CA-125 value was 869.8 U/ml, with a range between 10 and 10,000. Logistic regression analyses showed a positive relationship between CA-125 ≥ 500 U/ml and diaphragmatic carcinomatosis (OR 3.53, 95% CI 1.88–6.65, p < 0.01) and a positive relationship with residual disease (OR 2.46, 95% CI 1.37–4.41, p < 0.002). The positive relationship was maintained in the multivariate logistic regression analyses. After adjusting for age and ECOG status, CA125 ≥ 500 U/ml was positively related to diaphragmatic carcinomatosis (OR 3.51, 95% CI 1.86–6.64, p < 0.01) and residual disease (OR 2.41, 95% CI 1.33–4.38, p = 0.004).
Survival analyses
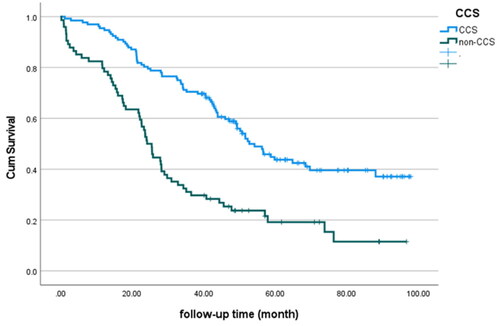
The median OS of the entire cohort was 43.9 months. Survival differences were observed between the CCS group and the non-CCS group (56.6 months and 25.5 months, respectively, p < 0.000) (). Both univariate and multivariate Cox regression analyses adjusted for age and ECOG performance status showed a positive relationship between the completeness of surgery and OS (HR 2.8; 95% CI 1.93–4.06, p < 0.01, and HR = 2.2; 95% CI = 1.58–3.35, p < 0.01, respectively).
Figure 1. Kaplan–Meier analysis of overall survival as shown for CCS or non-CCS in the whole patient group (p < 0.000).
When data were matched for CT-CPLNs, patients with an enlarged CPLN on CT scan vs. patients without an enlarged CT-CPLN, no differences in median survival were found (47.1 months vs. 41.6 months, p < 0.415). These results were sustained in both univariate and multivariate Cox regression analyses, adjusted for age, ECOG performance and residual disease at the end of surgery (HR = 1.17; 95% CI = 0.81–1.69, p = 0.415, and HR 0.995; 95% CI= 0.64-1.42, p = 0.819, respectively).
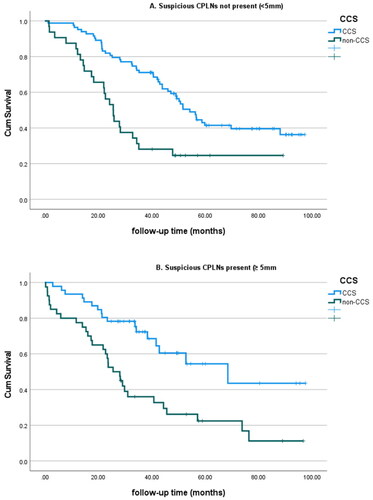
Kaplan–Meier analysis of the effect of completeness of surgery (CCS vs. non-CCS) showed significant differences in overall survival in both groups, as shown in . The median survival for patients with a CT-CPLN <5 mm was 56.5 months for patients who underwent CCS and 25.5 months for patients with residual disease (p < 0.01). For patients with a CT-CPLN ≥5 mm, the median survival was 68.4 months for the CCS group and 25.5 months for patients with residual disease (p = 0.001).
Figure 2. Overall survival according to CCS/non-CCS reported in the presence or absence of suspicious CT-CPLNs (defined as a short axis ≥5 mm).
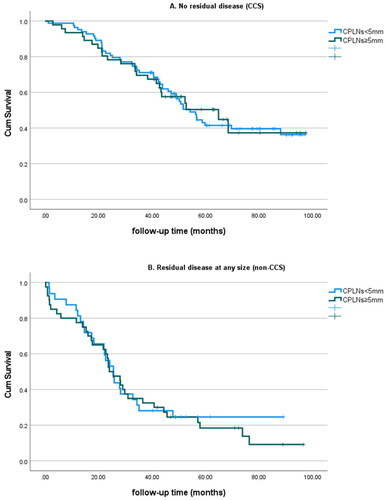
When data were matched for CCS vs. non-CCS, no differences in survival were found between patients with a CT-CPLN below or above 5 mm. In the CCS group, the patients exhibiting enlarged CT-CPLNs had a median survival of 56.5 months, compared with the median survival in patients with CT-CPLNs <5 mm (68.4 months) (p = 0.865). No differences in survival were registered in the non-CCS group (25.5 months vs. 25.5 months, for patients with CT-CPLNs ≥5 mm and <5 mm, respectively, p = 0.793) ().
Figure 3. Overall survival according to the presence/absence of suspicious CT-CPLNs and residual disease at the end of the surgery (CCS/non-CCS).
Univariate Cox regression analyses showed a negative correlation between CA-125 and OS. Every doubling of CA-125 increased the hazard of dying by 70% (HR = 1.70; 95% CI = 1.123–2.35, p = 0.001). The positive correlation was not maintained when data were adjusted for age, ECOG performance status and RD at the end of the surgery (HR = 1.29; 95% 0.94–1–76, p = 0.116).
Discussion
In this study, RD after surgery was found to be correlated with the presence of radiologically enlarged CPLNs and the CA-125 level. Patients with radiologically enlarged CPLN did not have worse OS than those not presenting enlarged CT-CPLN.
One of the most important prognostic factors in advanced ovarian cancer that might be influenced is the completeness of resection, and our results conform to this [Citation3]. The preoperative estimation of the tumor extent and the preoperative prediction of resectability are important factors that assist the surgeon in correctly selecting candidates for upfront surgery.
In patients with AOC, the tumor may extend extra abdominally, such as in CPLNs. There is an ongoing discussion about the impact of CPLNs as a predictor of tumor extent and resectability. In a study by Luger et al. a positive relationship was found between CPLNs ≥5 mm and tumor extent in the upper abdomen and surgical outcome [Citation8,Citation15]. These results are in line with the results of the present study.
Regarding the surgical removal of CPLNs, many questions remain to be answered. Surgical extirpation of CPLNs has been shown to be feasible and without considerable added morbidity, but it does not seem to improve survival [Citation13,Citation16]. Mert et al. analyzed 253 patients and found that survival was impaired in patients with enlarged CT-CPLNs and zero residual disease, whereas in patients with residual disease, no difference in survival was found regardless of the CT-CPLN status [Citation12]. In our study, we found no relationship between CT-CPLN status and survival, regardless of the completeness of cytoreductive surgery. This is in accordance with a previous study conducted by Sartor et al. in which no significant correlation between CT-CPLNs and survival was found (completeness of surgery was not considered in this study) [Citation17]. This might also explain why no improvement in survival was seen when CPLNs were removed. To the best of our knowledge, the present study is one of the few studies not showing a relationship between CT-CPLNs and survival regardless of surgical results. In addition to previous studies showing no survival benefit of removing CPLNs, the results of our study underline the lack of evidence supporting surgical removal, which subjects patients to unnecessary surgery.
It is worth to point out that some of the patients in our study may have been understated. There is 43% of patients with enlarged CT-CPLN without any confirmed histology as they were not removed. In the case that these were histologically positive CPLN they would belong to the stage IV group.
The impact of lymph node metastases in AOC is not clear. The Lymphadenectomy in Ovarian Neoplasms (LION) study [Citation18] has examined the role of iliac and paraaortic lymphadenectomy in advanced ovarian cancer patients. When complete cytoreductive surgery was achieved, the surgery was followed by intraoperative randomization of the patients to either systematic lymph node dissection or extirpation of bulky nodes. The results did not show any benefit of systematic lymph node extirpation. Hjerpe et al. explored the prognostic implication of nonregional lymph nodes as only distant metastatic sites in stage IV patients, concluding that these patients lived longer than stage IV patients with other stage IV manifestations [Citation19].
The prognostic role of enlarged lymph nodes in AOC seems to be limited for both intra-abdominal and extra-abdominal nodes, which emphasizes the importance of primary removal of abdominal disease, regardless of lymph node status. Interestingly, in the present study, no positive CT-CPLN was found in any of the stage IIIA cases. This adheres to the theory that the CPLN metastasis process depends on lymphatic drainage from the upper abdomen and diaphragm and not on the main lymphatic pathways and that it seems to be a surrogate marker of tumor load and upper abdominal disease [Citation20,Citation21].
CA-125 is a biomarker commonly used when diagnosing AOC. Studies on CA-125 as a reliable marker for predicting both tumor extent and resectability have shown contradictory results [Citation22]. In the present study, CA-125 was found to be correlated with both peritoneal carcinomatosis on the diaphragm and RD. For patients with a CA-125 value above 500 U/ml, the risk of diaphragmatic carcinomatosis was 3.5 times higher, and the risk for residual disease of any size was increased by 2.4 times. The prognostic role of CA-125 is still unclear; some studies have shown that CA-125 is a prognostic factor for recurrence but not for survival, whereas others have found that it is predictive of RD and survival [Citation23,Citation24]. In the present study, we found that CA-125 seems to play a prognostic role. The risk of dying of the disease increased by 70% for every doubling of the CA-125 value, but after adjusting for patient age, ECOG performance status and completeness of surgery, the correlation was no longer sustained.
In conclusion, CT-CPLNs and CA-125 could potentially be part of preoperative therapy planning management and are among the many predictive factors of surgical outcome. Regardless of CT-CPLN status, the only important independent prognostic factor in AOC seems to be the removal of all macroscopic disease.
Author contributions
Alba Plana: writing original draft and review and editing; Robert Talo: investigation; Nils-Olof Wallengren: investigation; Sonja Pudaric: investigation; Hanna Sartor: writing – review and editing; Mihaela Asp: conceptualization, methodology, formal analysis, investigation, writing - original draft and review and editing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available on request from the corresponding author, AP. The data are not publicly available due to their containing information that could compromise the privacy of research participants.
Additional information
Funding
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108.
- Colombo N, Sessa C, Du Bois A, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease†. Ann Oncol. 2019;30(5):672–705.
- Du Bois A, Reuss A, Pujade-Lauraine E, et al. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the arbeitsgemeinschaft gynaekologische onkologie studiengruppe ovarialkarzinom (AGO-OVAR) and the groupe d‘Investigateurs nationaux pour les etudes des cancers de l‘Ovaire (GINECO). cancer. Cancer. 2009;115(6):1234–1244.
- Stuart GC, Kitchener H, Bacon M, et al. 2010 Gynecologic cancer InterGroup (GCIG) consensus statement on clinical trials in ovarian cancer: report from the fourth ovarian cancer consensus conference. Int J Gynecol Cancer. 2011;21(4):750–755.
- Reuss A, Du Bois A, Harter P, et al. TRUST: trial of radical upfront surgical therapy in advanced ovarian cancer (ENGOT ov33/AGO-OVAR OP7). Int J Gynecol Cancer. 2019;29(8):1327–1331.
- Tsili AC, Naka C, Argyropoulou MI. Multidetector computed tomography in diagnosing peritoneal metastases in ovarian carcinoma. Acta Radiol. 2021;62(12):1696–1706.
- Caramella C, Pottier E, Borget I, et al. Value of cardiophrenic angle lymph node for the diagnosis of colorectal peritoneal carcinomatosis. Eur J Cancer. 2013;49(18):3798–3805.
- Luger AK, Steinkohl F, Aigner F, et al. Enlarged cardiophrenic lymph nodes predict disease involvement of the upper abdomen and the outcome of primary surgical debulking in advanced ovarian cancer. Acta Obstet Gynecol Scand. 2020;99(8):1092–1099.
- Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996;82:359–374.
- Kolev V, Mironov S, Mironov O, et al. Prognostic significance of supradiaphragmatic lymphadenopathy identified on preoperative computed tomography scan in patients undergoing primary cytoreduction for advanced epithelial ovarian cancer. Int J Gynecol Cancer. 2010;20(6):979–984.
- Suidan RS, Ramirez PT, Sarasohn DM, et al. A multicenter prospective trial evaluating the ability of preoperative computed tomography scan and serum CA-125 to predict suboptimal cytoreduction at primary debulking surgery for advanced ovarian, fallopian tube, and peritoneal cancer. Gynecol Oncol. 2014;134(3):455–461.
- Mert I, Kumar A, Sheedy SP, et al. Clinical significance of enlarged cardiophrenic lymph nodes in advanced ovarian cancer: implications for survival. Gynecol Oncol. 2018;148(1):68–73.
- Prader S, Vollmar N, Du Bois A, et al. Pattern and impact of metastatic cardiophrenic lymph nodes in advanced epithelial ovarian cancer. Gynecol Oncol. 2019;152(1):76–81.
- Forstner R, Sala E, Kinkel K, et al. ESUR guidelines: ovarian cancer staging and follow-up. Eur Radiol. 2010;20(12):2773–2780.
- Prader S, Harter P, Grimm C, et al. Surgical management of cardiophrenic lymph nodes in patients with advanced ovarian cancer. Gynecol Oncol. 2016;141(2):271–275.
- Salehi S, Mohammar R, Suzuki C, et al. Cardiophrenic lymph node resection in advanced ovarian cancer: surgical outcomes, pre- and postoperative imaging. Acta Oncol. 2018;57(6):820–824.
- Sartor H, Bjurberg M, Asp M, et al. Ovarian cancer subtypes and survival in relation to three comprehensive imaging parameters. J Ovarian Res. 2020;13(1):26.
- Harter P, Sehouli J, Lorusso D, et al. A randomized trial of lymphadenectomy in patients with advanced ovarian neoplasms. N Engl J Med. 2019;380(9):822–832.
- Hjerpe E, Staf C, Dahm-Kähler P, et al. Lymph node metastases as only qualifier for stage IV serous ovarian cancer confers longer survival than other sites of distant disease - a swedish gynecologic cancer group (SweGCG) study. Acta Oncol. 2018;57(3):331–337.
- Abu-Hijleh MF, Habbal OA, Moqattash ST. The role of the diaphragm in lymphatic absorption from the peritoneal cavity. J Anat. 1995;186(Pt 3):453–67.
- Holloway BJ, Gore ME, A'Hern RP, et al. The significance of paracardiac lymph node enlargement in ovarian cancer. Clin Radiol. 1997;52(9):692–697.
- Suidan RS, Ramirez PT, Sarasohn DM, et al. A multicenter assessment of the ability of preoperative computed tomography scan and CA-125 to predict gross residual disease at primary debulking for advanced epithelial ovarian cancer. Gynecol Oncol. 2017;145(1):27–31.
- Zwakman N, van de Laar R, Van Gorp T, et al. Perioperative changes in serum CA125 levels: a prognostic factor for disease-specific survival in patients with ovarian cancer. J Gynecol Oncol. 2017;28(1):e7.
- Piatek S, Panek G, Lewandowski Z, et al. Nadir CA-125 has prognostic value for recurrence, but not for survival in patients with ovarian cancer. Sci Rep. 2021;11(1):18190.
