Abstract
Background
Uveal melanoma is an orphan malignancy with very limited data on treatment options in metastatic setting.
Methods
In this single-center retrospective study, we describe real-world epidemiological and survival data on 121 metastatic uveal melanoma (MUM) patients registered in our institution. As a large tertiary referral center, almost 30% of all diagnoses in the Flemish region of Belgium were covered. Primarily, we determined whether introduction of immune checkpoint inhibitors (ICI) led to improved overall survival (OS) in MUM patients. Secondarily, response rates to ICI were assessed and we evaluated whether first-line ICI could be a valid alternative to liver-directed therapy (LDT) in liver-only disease.
Results
The initially perceived 10.8 months survival benefit from treatment with ICI disappeared after correction for immortality bias. By analyzing treatment type as time-varying covariate on OS, no significant benefit of ICI over other systemic therapies (HR = 0.771) or best supportive care (BSC) (HR = 0.780) was found. Also comparison of the pre-ICI versus ICI era showed no OS improvement after introduction of ICI in our center (p = 0.7994). Only liver-directed and local oligometastatic approaches were associated with a lower chance of mortality when compared to ICI (p = 0.0025), other systemic therapies (p = 0.0001) and BSC (p = 0.0003), yet without correction for selection bias. We reported overall response rates on ICI ranging from 8–15% and we found some support for neoadjuvant strategies with ICI resulting in remission or downsizing, allowing oligometastatic approaches later on. In first-line liver-only disease, median real-world progression-free survival and OS did not significantly differ between patients treated with LDT or ICI upfront (p = 0.2930 and p = 0.5461 respectively).
Conclusion
Although we documented responses to ICI, our analyses do not demonstrate an OS benefit of ICI over alternative treatment strategies for MUM. However, local treatment options, whether liver-directed or for oligometastatic disease, may be beneficial and should be considered.
Background
Uveal melanoma is the most common primary intraocular tumor in adults, but comprises less than 3% of all melanoma diagnoses [Citation1,Citation2]. With a worldwide incidence rate of approximately 5 per million adults per year, it is a rare disease [Citation3]. Similar to cutaneous melanoma, uveal melanoma arises from melanocytes, the body’s pigment producing cells. The choroid is the most common site of origin (>85–90%) within the uveal tract, followed by the ciliary body and the iris [Citation4]. Although fair skin that sunburns easily and light-colored eyes seem to be risk factors for its development [Citation5], the link between ultraviolet radiation exposure and development of uveal melanoma has not been validated in epidemiological studies so far [Citation6].
Patients may present with subtle changes in vision (floaters, flashes, blurry vision) as well as eye shape changes (dark spot on the pupil, altered pupil shape). At diagnosis, the majority of patients has local or locally advanced (extracapsular) disease and a seemingly favorable 5-year overall survival (OS) rate of 71–85% [Citation7], while 1–3% already have detectable synchronous metastases [Citation3]. However, 10–15 years after enucleation or brachytherapy, which are treatment options with similar oncological outcomes [Citation8], ∼50% of patients develop metastatic disease [Citation5]. Once disseminated, the prognosis is poor with an estimated median OS of 3–13 months and OS rates of only 43%, 13% and 1% at 1, 3 and 5 years respectively [Citation1,Citation3].
To date, very limited data are available on the efficacy of different treatment options for patients with metastatic uveal melanoma (MUM). Patients with exclusive and limited hepatic involvement can be treated with liver-directed therapies (LDT), with reported overall response rates (ORR) of 20–44% depending on treatment modality [Citation9]. For patients with widespread extrahepatic disease or patients ineligible for LDT, systemic treatment is required. However, uveal melanoma is generally refractory to conventional chemotherapy, such as dacarbazine, cisplatin, temozolomide, fotemustine, liposomal vincristine and paclitaxel [Citation10]. Also multikinase inhibitors are of limited use, since targetable mutations (e.g., BRAF) are typically absent [Citation3]. The role of immune checkpoint inhibitors (ICI), such as nivolumab and pembrolizumab (both PD-1 inhibitors) or ipilimumab (a CTLA-4 inhibitor) in uveal melanoma remains debated [Citation1,Citation3,Citation11–13]. Available survival data on ICI report median PFS of only 2.8–5.5 months [Citation1,Citation13,Citation14] and median OS ranging from 8.9–19.1 months [Citation1,Citation12–14].
Currently, the gp100 targeting T-cell receptor bispecific molecule Tebetafusp is the only drug displaying a significant OS benefit of 5.7 months over treatment of investigator’s choice in a phase 3 study with 378 treatment-naive MUM patients [Citation14]. Although promising, Tebetafusp can only be given to HLA-A*02:01 positive patients (including only 50% of the Caucasian and 35% of the African-American population [Citation15]). In addition, a significant proportion (44%) of patients experiences serious (≥ grade 3) treatment-related adverse events, mostly cytokine-release syndrome and skin-related events.
At present, no standard of care treatment for MUM patients has been established. Being a rare and difficult-to-treat disease, there is a paucity of prospective randomized controlled trials. Our group previously published the first Belgian study [Citation16] of 76 MUM patients diagnosed between 1957–2008. Median OS in this population was only 5 months, without any difference between treated and untreated patients. However, systemic treatment mainly consisted of chemotherapy (only 3% ICI), and LDT was never used. The aim of our current study was to explore the anti-tumor activity and potential role of ICI in MUM in a large population-based study.
Methods
Study design and definitions
For this single-center retrospective study, data of all 342 ocular melanoma patients diagnosed and treated in the University Hospitals Leuven between August 15th, 1992 and July 14th, 2021, were retrieved from the hospital-specific cancer registry and cross-referenced with the patient’s medical records. As a large tertiary referral center in Belgium, our institution provides care to uveal melanoma patients nationwide as well as internationally. As almost 30% of uveal melanoma diagnoses in the Flemish region of Belgium were included, we obtained representative real-world data on patients with this orphan malignancy. All patients aged >18 years diagnosed with metastatic disease during their disease course were selected. In total, 133 metastatic patients were identified (). Considering the differences in molecular profile and biological behavior, conjunctival (n = 8), orbital (n = 1) and patients with unknown site melanomas (n = 3) were excluded. Our study was approved by the Ethics Committee of University Hospitals Leuven (file number: MP017700).
The primary endpoint of the study was OS, which was calculated from first detection of metastatic disease until death or cencoring on August 1st, 2022. Secondary endpoints consisted of ORR and real-world progression-free survival (rwPFS). Exploratory endpoints were the ICI-related phenomenon of pseudoprogression, immune escape and treatment strategies in daily clinical practice. Responses were retrieved from patient files and could not be reassessed independently as not all imaging data were available due to software migration. In general, our center makes use of the Response Evaluation Criteria in Solid Tumors (RECIST1.1) guidelines to evaluate imaging data. Since time intervals between patient visits and imaging may be irregular in clinical practice and clinically relevant progression is also taken into account, rwPFS was evaluated. RwPFS was defined as the interval between initiation of treatment and recorded progression by the treating oncologist or death due to any cause. ORR was defined as the proportion of patients who achieved complete response (CR) or partial response (PR), while clinical benefit rate (CBR) was the percentage of patients with CR, PR or stable disease (SD). Pseudoprogression was defined as primary increase in tumor burden, followed by tumor regression upon treatment continuation. Best responses are reported.
Treatments were categorized as ICI, other systemic treatments, local treatments or best supportive care (BSC) (i.e., no anti-cancer treatment). Since ICI was introduced in our hospital as treatment for MUM on August 1st, 2010, we refer to the period before this date as the pre-ICI era and the period afterwards as the ICI era. All patients were observed until data cutoff on August 1st, 2022. The category ‘other systemic treatments’ included chemotherapy, tyrosine kinase inhibitors and Tebentafusp. Local treatments included LDT and local therapies such as surgery and external beam radiotherapy for oligometastatic disease. In our center, the selective internal radiation therapy (SIRT) procedure consisted of injection of radioactive 90Yttrium microspheres in the common/right/left hepatic artery or smaller branches (tumoricidal dose >100 Gy). For trans-arterial embolization small particles without chemotherapeutic impregnation were used. High dose melphalan was used for isolated hepatic perfusion. Combination of ipilimulab and nivolumab followed by nivolumab monotherapy was counted as one treatment line unless interrupted by local therapies. Consecutive treatments with different classes of ICI upon disease progression were counted as separate treatment lines.
Statistical analysis
Data analyses were performed using SAS software (version 9.4 of the SAS System for Windows). Cox regression was used to assess the association between treatment type and OS. Results are reported as hazard ratios (HR) with 95% confidence intervals (CI). As survival results from retrospective studies may be affected by immortality bias, the effect of treatment type on OS was assessed as a time-varying covariate. For this analysis, data were organized using a counting process data format with multiple rows per patient representing separate treatment periods. Results were corrected for extent of disease (liver-only, extrahepatic only or both) and interval to metastasis. Overestimation of other non-ICI treatments due to ongoing (possibly additive) effect of ICI after discontinuation was corrected with a sensitivity analysis, which censored patients at start of the next treatment. Possible continuous effects of ICI were investigated with ICI as a binary time-varying covariate that takes the value 0 during the period before a patient receives ICI and takes the value 1 from the moment ICI is started. It stays 1 even after termination of ICI and start of any other treatment.
In addition, survival in the pre-ICI versus ICI era was compared using Cox regression. The analysis was corrected for age and gender as the cohorts tended to differ on these characteristics. Patients in the pre-ICI era who eventually received ICI, were censored at the start of ICI. One patient on first-line ICI was censored as the type of ICI prior to referral was not documented. To investigate whether the benefit of certain treatment types would be more pronounced in liver-only disease, rwPFS and OS were calculated for this subgroup. One patient treated with SIRT followed by adjuvant ICI and 7 patients surgically treated for limited liver metastasis were excluded as they represent a group with a lower disease burden.
Results
Patient characteristics
Our study cohort consisted of 121 patients with uveal tract melanoma, the majority of which arose from the choroid (n = 80). 50% was male, median age at detection of metastasis was 66.1 years (range 35.7–91.3; ). Mutational status was not routinely examined, precluding assessment of association with treatment outcome. Almost all patients (90%) had local disease at time of initial diagnosis: 62% received surgery, 36% brachytherapy and 2% thermotherapy. Median interval from diagnosis to detection of metastasis was 29.9 months (range 0–305.4). The liver was the most frequently affected site (90%). Nevertheless, LDT was feasible in only 35 patients (29%). 34% of patients received multiple consecutive treatments, whereas 8 patients only received LDT, 15 only ICI and 29 only systemic treatment other than ICI. A substantial proportion of patients (22%) did not receive any anti-cancer treatment. Treatments installed in each line are depicted in Supplemental Figure 1. Median follow-up duration was 48.1 months (range 1.9–313.5) and median OS 9.3 months (range 0.1–104.6). By August 2022, 110 patients had died and 5 patients were lost to follow-up after a median of 46.8 months. Only six patients were alive at cencoring (median OS 35.6 months, range 17.7–65.1), 5 of whom interestingly had an ongoing response following ICI, while one 89-year-old patient with upfront multiple lung metastases survived for at least 34.1 months with BSC only. In total, 69 (63%) cancer-related deaths were recorded and at least 24 patients (22%) died of liver failure. Cause of death was unknown in 35 patients.
Table 1. Patient and tumor characteristics.
Overall survival
Uncorrected survival analysis indicated that patients who received ICI during their disease course had a longer median OS (17.8 months, interquartile range (IQR) 8.7–37.7) than patients who were never treated with ICI (7.0 months, IQR 3.2–14.6). However, upon correction for immortality bias and after correction for confounders (‘extent of disease’ and ‘interval to metastasis’), no significant benefit of ICI over other systemic therapies (HR = 0.771) or BSC (HR = 0.780) could be established (). No significant additional or synergistic effect of prior ICI on next treatment lines was established in the sensitivity analysis and cox regression analysis to capture possible continued ICI effects after treatment cessation did not alter OS results either. All assessments showed that only LDT and local treatment of oligometastatic disease were associated with a significantly lower chance of mortality.
Table 2. Overall survival by treatment type as time-varying covariate (n = 121).
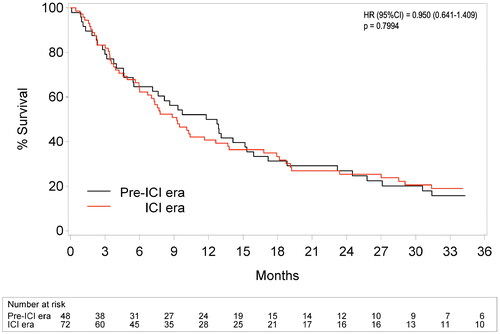
Also, comparison of the pre-ICI versus ICI era confirmed that introduction of ICI did not result in improvement of OS in our center (p = 0.7994, ) even though 42/72 (58%) patients were treated with ICI in this time period. Number of treatment lines was similar in both eras (p = 0.636) but in the ICI era, patients received significantly more local therapies (p = 0.015) and more patients were treated exclusively locally (p = 0.084).
Figure 1. Overall survival in pre-ICI versus ICI era. ICI: immune checkpoint inhibitors. HR: hazard ratio. CI: confidence interval. Patients were assigned to the pre-ICI or ICI era if first metastatic disease was diagnosed respectively before or after introduction of immune checkpoint inhibitors in our center (i.e., August 1st, 2010). Cox regression analysis was corrected for age and gender, as both cohorts tended to differ on these characteristics.
Efficacy of immune checkpoint inhibitors
In our study cohort, 46 (38%) patients received 66 ICI treatment lines consisting of PD1-inhibitors and CTLA4-inhibitors in combination or (consecutive) monotherapy. Recorded ORR in first line (14%) was similar to second line (11%) and above (12%). ORR was 15% for PD1-inhibitors in monotherapy, while only 8% for CTLA-4 inhibitors in monotherapy. For combination therapy, ORR of 14% was reached (Supplemental Table 3). ICI was generally initiated as palliative treatment, although in 9 cases LDT could be applied after ICI treatment. For some patients, response evaluation was challenging: after initial (pseudo)progression by RECIST1.1, CR was obtained upon treatment continuation. Others demonstrated (partially) refractory disease and could be salvaged with subsequent local treatments ().
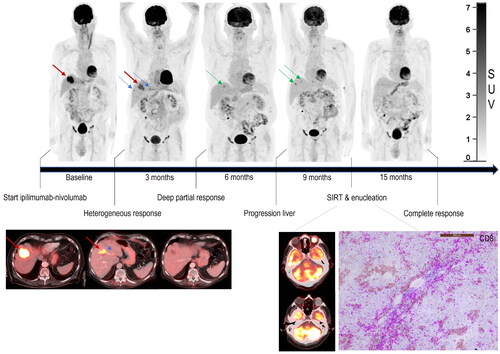
Figure 2. Response patterns to immune checkpoint inhibitors. Combination therapy with ipilimumab and nivolumab resulted in a complete remission of extrahepatic nodal and peritoneal metastases on 18F-FDG PET-CT. Response in the liver was heterogeneous, with regression of some liver metastases while others progressed. Similarly, intra-ocular tumor infiltrating CD8-positive T-cells were not able to induce a response in the eye. Nevertheless, neoadjuvant immune checkpoint inhibitor treatment allowed some patients to proceed with local therapy for refractory disease, which resulted in prolonged progression-free survival and possibly cure.
First-line treatment for liver-only disease
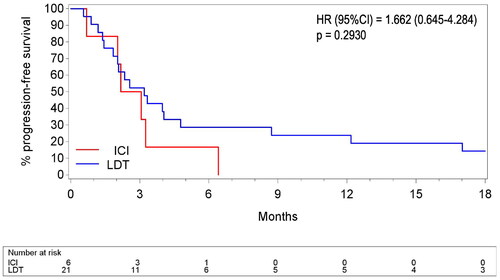
At diagnosis of metastatic disease, 72 patients (60%) had liver involvement only. In this cohort, median rwPFS did not differ significantly between patients who received LDT (n = 21) and those who received ICI (n = 6) in first line (3.2 versus 2.6 months respectively, p = 0.2930; ), eventhough the ICI group contained patients with a higher disease burden. Median rwPFS for other systemic therapies was merely 1.6 months (n = 21). Half of the LDT patients (n = 10) went on to treatment with ICI at some point and vice versa, 3 ICI patients received LDT later on during their disease course. Median OS was 8.8 versus 18.2 months for ICI and LDT in first line respectively, but this difference was not statistically significant (p = 0.5461; Supplemental Table 4).
Figure 3. Real-world progression-free survival in liver-only disease. ICI: immune checkpoint inhibitors: pembrolizumab, nivolumab and ipilimumab, as monotherapy or as combination therapy. LDT: liver-directed therapies: selective internal radiation therapy, trans-arterial liver embolization, liver perfusion with melphalan and total liver external beam radiotherapy. "Surgical resection of liver metastasis" was excluded for this analysis as it reflects a different patient group with (very) low hepatic disease burden.
Discussion
In this large single center real-world cohort study of 121 MUM patients, spanning a time period of 30 years, we found a median OS of 9.3 months, which is in accordance with the 3–13 months reported in literature [Citation1,Citation3].
Establishing treatment efficacy in rare metastatic malignancies is challenging, especially when biological behavior is diverse as in uveal melanoma. Patients with more indolent tumors may receive more treatment lines which do not necessarily translate into better quality of life or survival benefit. Indeed, the initially perceived ∼11 months survival benefit from treatment with ICI was not existing after thorough correction for biases and confounders. We corrected for immortality bias both by analyzing ICI as a time-varying covariate on OS and by comparing the pre-ICI and ICI era. As treatment choice may have been influenced by patient characteristics (i.e., Karnofsky performance score, comorbidities, cancer burden and disease localization), different time eras (in which similar known and unknown baseline characteristics can be assumed) were compared. This minimized selection bias, but did not correct for random error. The 1-year OS rate of our patients in the ICI era (40.7%) was similar to that reported in the large meta-analysis (including 912 patients) of Khoja et al. (43%) [Citation1], but was not significantly longer compared to the pre-ICI era. In our analyses of ICI as a time-varying covariate, no survival advantage of ICI could be established either over other systemic treatments or BSC.
This is in accordance with the observed low ORR for ICI in our trial (8–15% for monotherapy and 14% for combination therapy). In literature, reported response rates to ICI in uveal melanoma (around 4–10% in monotherapy [Citation11,Citation17,Citation18] and 12–21% in combination therapy [Citation11,Citation12,Citation18–20]) are much lower compared to cutaneous melanoma, where response rates of 44% with monotherapy and up to 58% with combination therapy are achieved [Citation21]. The difference in response could be attributed to the extremely low mutational burden in uveal melanoma (0.5 mt/Mb compared to 49.2 mt/Mb in cutaneous melanoma), its low PD-1/PD-L1 expression and the immune-privileged tumor microenvironment of UM cells, resulting in a roadblock for ICI [Citation22,Citation23].
In general, our analyses do not support a strong recommendation for ICI as standard treatment for MUM. The same conclusion was made in other retrospective population studies [Citation17,Citation19,Citation20] and two recent large meta-analyses [Citation1,Citation3]. On the contrary, the Danish cohort study of Bol et al. [Citation11] did show a significantly improved long-term survival with the introduction of ICI, although mainly in patients who received ICI as first-line treatment. This could explain the difference with our results as in our ICI era cohort only 20/72 (28%) of patients were treated with first-line ICI (vs 87% in the Danish study). Also, significantly more patients received local therapies in our study, whereas in the study by Bol et al. [Citation11] patients receiving these treatments were excluded from analysis. Two recent prospective phase-2 studies [Citation12,Citation13] showed activity of ICI (ipilimumab-nivolumab combination therapy) in MUM as well, with some deep and sustained responses.
So, to date, treatment with ICI in MUM remains a controversial yet unresolved topic. Despite rather limited responses, no better treatment options are available for approximately 50% of patients with widespread metastatic disease (i.e., those ineligible for Tebentafusp). Since ICI is considered to be a treatment option with a manageable toxicity profile (at least anti-PD1 in monotherapy), that could possibly induce long-term disease control in responding patients. Future trials are warranted to better select patients and identify specific biomarkers for treatment response [Citation24].
Back in 2005, Rietschel et al. [Citation25] already suggested the existence of 2 subsets of MUM patients with different tumor biology, i.e., a minority with a more indolent disease course (median OS > 4 years) versus a larger group with more aggressive behavior (median OS of only 12 months). Characteristics correlated with prolonged survival were extrahepatic disease (site of first metastasis being lung or soft tissue), female gender, age younger than 60 years and longer interval from diagnosis to metastasis. A special role for ICI in patients with extrahepatic disease was also suggested by Rossi et al. [Citation26], due to the supposed immune escape in patients with liver metastasis only (i.e., the ‘liver tolerance effect’ [Citation27]). In our analysis, we examined these variables as well, but no predictive value was found (Supplemental Table 2).
Interestingly, despite general low immunogenicity of UM, the phenomenon of pseudoprogression was observed in several cases, indicating strong mobilization of tumor infiltrating T-cells. In addition, 7 mixed responses to ICI were observed indicating clonal evolution. Whether somatic pathogenic mutations in MBD4 [Citation28] contributed to ICI sensitivity in these patients could not be tested as not all metastatic lesions were routinely biopsied. Understanding of the mechanism which increases immunogenicity and overcomes resistance to ICI may result in new treatment strategies, but further research in this domain is needed.
Liver-directed and local oligometastatic approaches were the only treatments in our study cohort associated with a significant survival benefit (compared to ICI: p = 0.0025; other systemic therapies: p = 0.0001; and BSC only: p = 0.0003), even after correction for immortality bias. Although selection bias should be taken into account, i.e., reflection of the better prognosis of patients with low disease burden as they are selected for local treatment, it does not exclude that, if deemed possible, an oligometastatic approach could improve survival in selected patients. Nevertheless, no prolonged survival was demonstrated in the ICI era, although significantly more local therapies were applied.
To overcome resistance and enhance the efficacy of ICI, currently ongoing trials focus on combining ICI with novel immunologic strategies [Citation24] and even with liver-directed therapies [Citation23,Citation27].
We found some support for neoadjuvant strategies in 12 patients, allowing local liver-directed or oligometastatic treatment approaches after downsizing. Furthermore, we demonstrated that treatment with ICI may result in complete remission of extrahepatic metastasis allowing subsequent LDT for refractory liver metastasis resulting in a prolonged disease-free survival (>33 months; ).
Also, adjuvant ICI strategies have been explored. Levey et al. [Citation29] demonstrated a significant OS advantage of 16.5 months in a retrospective review of 24 histologically proven ocular melanoma patients with hepatic metastasis when ICI was administered within 3 months of hepatic embolization with 90Y-microspheres. The randomized phase II CHOPIN trial [Citation30], which is enrolling MUM patients with unresectable liver metastases with or without extrahepatic disease in the Netherlands, is investigating whether a synergistic effect might be induced by ICI following locoregional treatment of liver metastases. Hypothesis for this strategy is that tumor necrosis caused by LDT, generates an anti-tumor response by increasing the expression of tumor-associated antigens and the recruitment of tumor infiltrating lymphocytes, which can be further boosted by the addition of ICI.
Finally, no difference between first-line LDT and ICI was found regarding rwPFS in liver-only disease and order of treatment did not seem to affect the median OS. So, both strategies are warranted and first-line therapy can be tailored to individual patients’ needs taking into account comorbidity and the risks of liver failure due to progressive liver metastasis or immune-related hepatitis.
Despite the limitations inherent to the retrospective design, strengths of our study are the large and representative series of 121 MUM patients, reflecting almost 30% of uveal melanoma diagnoses in the Flemish part of Belgium and spanning a time period of 30 years. We provided real-world data on the anti-tumor activity and potential role of ICI, after extensive correction for immortality bias and confounders. Also inclusion of local and liver-directed approaches, for which there is emerging positive data from clinical trials [Citation24,Citation27,Citation29], is a strength compared to earlier real-world series on ICI in MUM.
Conclusion
Since in this large real-world cohort study, no proven survival benefit of ICI was found, our analyses do not support a strong recommendation for ICI as standard treatment for MUM. A selected group of patients however may benefit from ICI treatment strategies resulting in some deep and sustained responses. Also local treatment, whether liver-directed or for oligometastatic disease, may be beneficial even in case of extrahepatic disease and should be considered. Given the poor prognosis of metastasized patients, prospective randomized controlled trials on ICI and LDT are urgently needed and should include assessment of prognostic and predictive factors to allow better selection of patients who might benefit from these (combination) strategies.
Supplemental Material
Download MS Word (49.5 KB)Supplemental Material
Download MS Word (52.8 KB)Acknowledgements
The authos thank Brigitte Bankaer for her administrative support. Statistical analyses were done by Anouschka Laenen and financed by PMC, FJSHW and the Leuven Cancer Institute.
Disclosure statement
PMC has received study budget funds from AstraZeneca; was advisory board member for AbbVie, AstraZeneca, Bayer, Bristol Myers Squibb, Daiichi-Sankyo, Leo Pharma, Merck Serono, MSD, and Vifor Pharma. CMD has been a consultant for Terumo, Ipsen, Sirtex, Bayer and PSI CRO. DRT received speaker honorary from Novartis Pharma AG (Switzerland) and Biogen (USA), travel reimbursement from GE-Healthcare (UK) and UCB (Belgium) and collaborated with Novartis Pharma AG (Switzerland), Probiodrug (Germany), GE-Healthcare (UK), and Janssen Pharmaceutical Companies (Belgium). DRT receives grants from Fonds Wetenschappelijk Onderzoek (FWO (Vlaanderen): G0F8516N, G065721N), Stichting Alzheimer Onderzoek (SAO-FRA (Belgium): 2020/017), and KU-Leuven Internal Funding (C14/17/107; C14/22/132; C3/20/057). Other authors have no competing interests to disclose with regard to this study.
Data availability statement
The data that support the findings of this study are available from the corresponding authors, LV, PMC or FJSHW, upon reasonable request. The data are not publicly available because they contain information that could compromise the privacy of research participants.
Additional information
Funding
References
- Khoja L, Atenafu E, Suciu S, et al. Meta-analysis in metastatic uveal melanoma to determine progression free and overall survival benchmarks: an international rare cancers initiative (IRCI) ocular melanoma study. Ann Oncol. 2019;30(8):1370–1380.
- National Organization for Rare Disorders. Rarediseases.org [Internet]. Ocular melanoma. 2018. https://rarediseases.org/rare-diseases/ocular-melanoma
- Rantala ES, Hernberg MM, Piperno-Neumann S, et al. Metastatic uveal melanoma: the final frontier. Prog Retin Eye Res. 2022;90:1–53.
- Valasapalli S, Guddati AK. Nation-wide trends in incidence-based mortality of patients with ocular melanoma in USA: 2000 to 2018. Int J Gen Med. 2021;14:4171–4176.
- Patel DR, Patel BC. Ocular melanoma. Treasure Island (FL): statPearls Publishing; 2022.
- Pane A, Hirst L. Ultraviolet light exposure as a risk factor for ocular melanoma in Queensland, Australia. Ophthalmic Epidemiol. 2000;7(3):159–167.
- American Society of Clinical Oncology. Cancer.net [Internet]. Eye Cancer. 2020. [cited 2022 Aug 6]. Available from: https://www.cancer.net/cancer-types/eye-cancer/view-all.
- Diener-West M, Earle JD, Fine SL, et al. The coms randomized trial of iodine 125 brachytherapy for choroidal melanoma, III: initial mortality findings. COMS report no. 18. Arch Ophthalmol. 2001;119(7):969–982.
- Rowcroft A, Loveday BPT, Thomson BNJ, et al. Systematic review of liver directed therapy for uveal melanoma hepatic metastases. HPB. 2020;22(4):497–505.
- Dhamani AM, Shaikh IN, Advani S, et al. Redefining the treatment of metastatic uveal melanoma with immunotherapy – A case report. IJMIO. 2022;7(2):46–49.
- Bol K, Ellebaek E, Hoejberg L, et al. Real-world impact of immune checkpoint inhibitors in metastatic uveal melanoma. Cancers. 2019;11(10):1489.
- Pelster M, Gruschkus S, Bassett R, et al. Nivolumab and ipilimumab in metastatic uveal melanoma: results From a Single-Arm phase II study. J Clin Oncol. 2021;39(6):599–607.
- Piulats J, Espinosa E, de la Cruz Merino L, et al. Nivolumab plus ipilimumab for treatment-naïve metastatic uveal melanoma: an open-label, multicenter, phase II trial by the Spanish multidisciplinary melanoma group (GEM-1402). J Clin Oncol. 2021;39(6):586–598.
- Nathan P, Hassel JC, Rutkowski P, et al. Overall survival benefit with tebentafusp in metastatic uveal melanoma. N Engl J Med. 2021;385(13):1196–1206.
- Olivier T, Prasad V. Tebentafusp in first-line melanoma trials: an outperforming outlier. Transl Oncol. 2022;20:4–7.
- Cerbone L, Van Ginderdeuren R, Van Den Oord J, et al. Clinical presentation, pathological features and natural course of metastatic uveal melanoma, an orphan and commonly fatal disease. Oncol. 2014;86(3):185–189.
- Algazi A, Tsai K, Shoushtari A, et al. Clinical outcomes in metastatic uveal melanoma treated with PD-1 and PD-L1 antibodies. Cancer. 2016;122(21):3344–3353.
- Zhao L, Xia W, Zhang Y, et al. Efficacy and safety of immune checkpoint blockades in the treatment of ocular melanoma: a systematic review and meta-analysis. Front Oncol. 2021;11:1–10.
- Heppt MV, Amaral T, Kähler KC, et al. Combined immune checkpoint blockade for metastatic uveal melanoma: a retrospective, multi-center study. J Immunother Cancer. 2019;7(1):1–9.
- Najjar YG, Navrazhina K, Ding F, et al. Ipilimumab plus nivolumab for patients with metastatic uveal melanoma: a multicenter, retrospective study. J Immunother Cancer. 2020;8(1):e000331.
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34.
- Peterson C, Denlinger N, Yang Y. Recent advances and challenges in cancer immunotherapy. Cancers (Basel). 2022;14(16):3972.
- Szeligo BM, Ivey AD, Boone BA. Poor response to checkpoint immunotherapy in uveal melanoma highlights the persistent need for innovative regional therapy approaches to manage liver metastases. Cancers (Basel). 2021;13(14):3426.
- Komatsubara K, Carvajal R. Immunotherapy for the treatment of uveal melanoma: current status and emerging therapies. Curr Oncol Rep. 2017;19(7):45.
- Rietschel P, Panageas KS, Hanlon C, et al. Variates of survival in metastatic uveal melanoma. J Clin Oncol. 2005;23(31):8076–8080.
- Rossi E, Croce M, Reggiani F, et al. Uveal melanoma metastasis. Cancers (Basel). 2021;13(22):5684.
- Blomen C, Kött J, Hartung T, et al. Combination of immune checkpoint inhibitors and liver-specific therapies in liver-metastatic uveal melanoma: can we thus overcome its high resistance? Cancers (Basel). 2021;13(24):6390.
- Rodrigues M, Mobuchon L, Houy A, et al. Outlier response to anti-PD1 in uveal melanoma reveals germline MBD4 mutations in hypermutated tumors. Nat Commun. 2018;9(1):1–6.
- Levey AO, Elsayed M, Lawson DH, et al. Predictors of overall and Progression-Free survival in patients with ocular melanoma metastatic to the liver undergoing Y90 radioembolization. Cardiovasc Intervent Radiol. 2020;43(2):254–263.
- Tong TML, van der Kooij MK, Speetjens FM, et al. Combining hepatic percutaneous perfusion with ipilimumab plus nivolumab in advanced uveal melanoma (CHOPIN): study protocol for a phase ib/randomized phase II trial. Trials. 2022;23(1):1–13.
