Abstract
Background
This dose-escalation study evaluated the toxicity and efficacy of different stereotactic body radiation therapy (SBRT) doses for selecting an optimal dose for prostatic adenocarcinoma (PCa).
Materials and methods
This clinical trial was registered at UMIN (UMIN000014328). Patients with low- or intermediate-risk PCa were equally assigned to 3 SBRT dose levels: 35, 37.5, and 40 Gy per 5 fractions. The primary endpoint was the occurrence rate of late grade ≥2 genitourinary (GU) and gastrointestinal (GI) adverse events at 2 years, while the secondary endpoint was the 2-year biochemical relapse-free (bRF) rate. Adverse events were evaluated using the Common Terminology Criteria for Adverse Events version 4.0.
Results
Seventy-five patients (median age, 70 years) were enrolled from March 2014 to January 2018, of whom 10 (15%) and 65 (85%) had low- and intermediate-risk PCa, respectively. The median follow-up time was 48 months. Twelve (16%) patients received neoadjuvant androgen deprivation therapy. The 2-year occurrence rates of grade 2 late GU and GI toxicities were 34 and 7% in all cohorts, respectively (35 Gy: 21 and 4%; 37.5 Gy: 40 and 14%; 40 Gy: 42 and 5%). The occurrence risk of GU toxicities significantly increased with dose escalation (p = 0.0256). Grades 2 and 3 acute GU toxicities were observed in 19 (25%) and 1 (1%), respectively. Grade 2 acute GI toxicity was observed in 8 (11%) patients. No grade ≥3 GI or ≥4 GU acute toxicity or grade ≥3 late toxicity was observed. Clinical recurrence was detected in 2 patients.
Conclusions
An SBRT dose of 35 Gy per 5 fractions is less likely to cause adverse events in patients with PCa than 375- and 40-Gy SBRT doses. Higher doses of SBRT should be applied with caution.
Introduction
Prostatic adenocarcinoma (PCa) is one of the most common cancers among men worldwide [Citation1]. Treatment options for PCa include surgery and external beam or interstitial radiation therapy (RT). Owing to technological advancements and the possibility of radiation dose escalation, treatment outcomes of RT for PCa have improved in recent years. Thus, RT is recognised as an effective and curative treatment option for men with localised PCa and has become comparable to surgical resection. Currently, various RT modalities have been developed and utilised for the treatment of PCa. A characteristic biological feature of PCa is its unique α/β ratio, which is even lower than surrounding normal tissues [Citation2]. Therefore, ultra-hypofractionated (high radiation dose, over 3.4 Gy per fraction) stereotactic body RT (SBRT) for PCa has attracted significant attention as a treatment modality for PCa, which can maximise therapeutic effect and minimise toxicities.
Although multiple clinical outcomes of SBRT for PCa have been reported, the applicability of SBRT is still considered controversial and has not been firmly stated in some practice guidelines [Citation3,Citation4]. In addition, there is scope for improvement in the treatment outcomes in patients with unfavourable intermediate- or high-risk PCa. Using a median dose of 36.25 Gy per 4–5 fractions, the 5-year biochemical relapse-free (bRF) survival rates after SBRT were 95%, 84% and 81% for low-, intermediate- and high-risk patients, respectively [Citation5]. To address this issue, dose escalation of RT may be promising, considering the favourable treatment outcomes achieved with high dose of brachytherapy (over biologically equivalent dose [BED] of 200 Gy at α/β of 2) in high-risk patients [Citation6]. Draulans et al. [Citation7] reported the feasibility of simultaneous focal boosting to a macroscopic tumour with acceptable toxicity and the applicability of SBRT for unfavourable intermediate- or high-risk PCa. However, drastic dose escalation of SBRT reportedly results in damages to the surrounding tissues. An SBRT dose of 50 Gy per 5 fractions (BED = 300 Gy at α/β of 2) reportedly resulted in increased grade ≥3 genitourinary (GU) or gastrointestinal (GI) toxicities [Citation8]. Thus far, the feasibility of dose escalation and optimal dose fractionation of SBRT, which can be used to achieve therapeutic effect and safety, remains controversial and requires evaluation. In this study, we compared three dose fractionation methods as follows: 35, 37.5, and 40 Gy per 5 fractions. The 35 Gy (BED of 158 Gy at α/β of 2) dose was similar to doses commonly used in external beam RT. The 37.5 Gy (BED of 178 Gy at α/β of 2) dose was an intermediate dose between the other two dose fractionations, and the 40 Gy (BED of 200 Gy at α/β of 2) dose was similar to doses commonly used in high-dose-rate brachytherapy. This dose escalation clinical trial investigated the toxicity and efficacy of different SBRT doses to determine a feasible and optimal SBRT dose for PCa.
Methods
Overview of the clinical trial
This was a single-institution, prospective, phase I/II dose escalation study (UMIN000014328) conducted from March 2014 to January 2018 in patients with PCa. We assessed the safety and efficacy of fiducial-based SBRT using CyberKnife (Accuray Inc., Sunnyvale, CA, USA) for localised low- or intermediate-risk PCa. The study protocol was approved by the Human Ethics Review Committee of the Osaka University Hospital and signed informed consent form was obtained from all patients.
We set 3 levels (35, 37.5, and 40 Gy per 5 fractions) of the examined RT dose, and intended to treat 25 patients in each dose level. Dose escalation to the next level was allowed if ≤7 of 25 patients experienced grade ≥2 GU or GI toxicities at the time of completion of registration at a given dose level and considering registration to the next dose level.
Endpoints
The primary endpoint was the 2-year occurrence rate of late grade ≥2 GU and GI adverse events, while the secondary endpoint was the 2-year bRF rate. Adverse events were evaluated using the Common Terminology Criteria for Adverse Events version 4.0 [Citation9]. Worsening of symptoms (such as urinary frequency or urinary retention), which were managed with continuous medication before SBRT, was defined as grade 2 toxicity. Biochemical relapse was defined based on the Phoenix definition (prostate-specific antigen [PSA] level >2 ng/mL from the nadir) [Citation10].
Patient eligibility criteria
The eligibility criteria included the following: histologically confirmed PCa by biopsy; no lymph node or distant metastases diagnosed by computed tomography (CT), magnetic resonance imaging (MRI), and bone scintigraphy (CT could be omitted if pelvic lymph nodes were evaluated by MRI); clinical T1c to T2c and N0 and M0 according to the Union for International Cancer Control 2009 TNM classification [Citation11]; Gleason’s score ≤7 (including 4 + 3); pre-treatment PSA level ≤20 ng/mL (in patients who underwent androgen deprivation therapy [ADT], the level before starting ADT was used); retaining sufficient major organ function (white blood cell count ≥3,000/μL, haemoglobin level ≥10.0 g/dL, platelet count ≥10 × 104/μL, creatinine level ≤2.0 mg/dL, glutamic-oxaloacetic transaminase level ≤100 U/L, glutamic pyruvic transaminase level ≤100 U/L, and no haemostatic dysfunction); age of at least 20 and <80 years at enrolment; provision of written informed consent.
The exclusion criteria were as follows: active double cancer; uncontrolled diabetes; use of anticoagulant or antiplatelet drugs that could not be stopped; severe cranial nerve disease; collagen vascular disease; prior pelvic surgery or RT; history of transurethral resection of the prostate; prostatic enlargement and lower urinary tract symptoms (International Prostate Symptom Score, which is a modification of the American Urological Association symptom index for benign prostatic hyperplasia ≥15) [Citation12]; no consent to or medically impossible to insert gold markers; ADT continued for >1 year before starting SBRT; castration-resistant prostate cancer (disease progression or PSA level increased >2 ng/mL from the nadir during ADT, with serum testosterone level <50 ng/dL).
Androgen deprivation therapy
Neoadjuvant ADT for 6 months was provided to intermediate-risk patients with ≥2 of the following factors: cT2b or T2c; initial PSA level >10 ng/mL; Gleason’s score of 3 + 4 or 4 + 3. Moreover, neoadjuvant ADT for the indicated patients was not imperative. Some eligible patients were allowed to refuse ADT. In addition, neoadjuvant ADT was allowed for patients without above-referenced factors who had initiated ADT in another institution before registration, provided that the duration period was within 1 year. None of the patients underwent adjuvant ADT.
Radiation therapy
Three gold fiducial markers were placed in the prostate for tracking. CT was then performed 1–2 weeks later. During planning of CT, a urinary catheter was inserted to visualise the urethra. After urinating immediately before a CT scan, 100 mL of saline was injected into the bladder, and the urinary catheter was clamped.
The clinical target volume (CTV) was set around the prostate (a 1-mm margin to the dorsal side and a 3-mm margin in other directions) and included the proximal region of the seminal vesicles (approximately 1 cm from the prostate). The planning target volume (PTV) was the CTV with a 2-mm margin in all directions. The defined organs at risk (OARs) were the rectum (delineated in CT slices in the range of 1 cm from the cranial and caudal ends of the PTV), whole bladder, urethra, and femoral heads. The prescribed dose of SBRT was 35, 37.5, or 40 Gy per 5 fractions to the lowest dose distributed to 95% volume of the PTV. The optimising procedure was conducted to set the prescribed isodose line to approximately 80% (70–90%), i.e., the maximum dose in the PTV was approximately 125% (111–143%) of the prescribed dose. The dose constraints for the OAR are listed in .
Table 1. Dose constraints of SBRT.
We confirmed the absence of relative displacement between the gold marker and the prostate or urethra via CT scanning before each treatment. The urinary catheter was placed continuously throughout the treatment period or inserted before each treatment, and urination was performed from the urinary catheter immediately before CT scanning. Subsequently, 100 mL of saline was injected into the bladder, and the urinary catheter was clamped. Irradiation was performed on 5 consecutive weekdays, and the total treatment period was 5–8 days.
Follow-up and evaluation
All patients were followed up 1 month after treatment with a medical examination and blood (complete blood count and assessment of liver and kidney function) and urine tests (qualitative urine tests and, if necessary, urine sediment examination). The medical examination and evaluation of PSA level were performed at 3, 6, 9, 12, 15, 18, 21, and 24 months. The blood and urine tests were performed 12 and 24 months post-treatment.
Statistical analyses
We used Tukey’s test to compare the clinical background and dose-volume parameters of SBRT between patients’ cohorts corresponding to different dose levels. The time to occurrence of toxicity or biochemical relapse was defined as the number of months from the initial day of SBRT to the day of the events. Occurrence rates of toxicity and bRF rate were calculated using the Kaplan–Meier method. The log-rank test for trend was used to examine whether there is a linear trend of occurrence rates of toxicity and bRF across patients’ cohorts. The Cox proportional hazards model was used to estimate the hazard ratio (HR) in terms of the difference in prescribed radiation dose and PTV with Dunnett’s test for multiple comparisons. Statistical analyses were performed using JMP Pro version 13 (SAS Inc., Cary, NC, USA) and SAS software version 9.4 (SAS Inc., Cary, NC, USA). Statistical significance was set at p < 0.05.
Results
Patients’ characteristics, dose-volume parameters, and clinical outcomes
A total of 75 consecutive patients with localised low- or intermediate-risk PCa (according to the National Comprehensive Cancer Network guideline [Citation13]) were registered in this study. Dose-limiting toxicity was not observed in more than the predetermined number of patients when we intended to move on to the next dose level. We treated 25 patients at each dose level, as planned. Patients’ characteristics are listed in . The clinical backgrounds of the patients were not significantly different among the 3 cohorts.
Table 2. Patients’ characteristics and DVH parameters.
Prostate volume and PTV were significantly larger in the 37.5-Gy cohort than in the other cohorts (p = 0.0084 and p = 0.0007, respectively). Among the dose-volume parameters of SBRT, D2cc of the rectum (p = 0.0011), D10cc of the bladder (p = 0.0018), D10% (p < 0.0001), and D30% of the urethra (p < 0.0001) significantly increased and correlated with dose escalation of SBRT. The dose-volume parameters are summarised in .
Biochemical relapse was observed in 5 patients during follow-up, and the 2-year bRF rate was 96%. Among these 5 patients, clinical recurrence was identified in 2:1 with local recurrence while the other with lymph node and bone metastatic recurrence. In another patient, the PSA level exceeded 2 ng/mL from the nadir within 2 years. He was diagnosed with prostatitis, and his PSA level gradually decreased after medication for prostatitis without any cancer treatment. Therefore, we did not consider this transition at PSA level as a biochemical relapse. One patient, who had not been diagnosed with the disease at registration to the trial, died of myelodysplastic syndrome 24 months after SBRT. All other patients were alive at their last follow-up.
Acute toxicity
Grade 3 acute urinary tract infection was reported in 1 (1%) patient who underwent SBRT of 40 Gy. Grade 2 acute GU toxicities were reported in 19 patients (25%). Of these 19 patients, 6, 8, and 5 received SBRT of 35, 37.5, and 40 Gy, respectively. Grade 2 acute GI toxicities were reported in 8 (11%) patients. Of these 8 patients, 1, 3, and 4 received SBRT of 35, 37.5, and 40 Gy, respectively. Detailed descriptions of the toxicities are provided in . There was no acute grade 4 or 5 GU and grade > 3 GI toxicity.
Table 3. Summary of grade ≥2 toxicities.
Late toxicity
Late GU and GI toxicities were observed in 47 (63%) (median time to onset, 12 months) and 20 (27%) (median time to onset, 8.5 months) patients, respectively. Grade 2 late GU toxicities were observed in 29 (37%) patients (median time to onset, 12 months), and grade 2 GI toxicities were observed in 6 (8%) patients (median time to onset, 8.5 months). There was no grade >3 late GU or GI toxicities. A summary of grade 2 late toxicities is presented in .
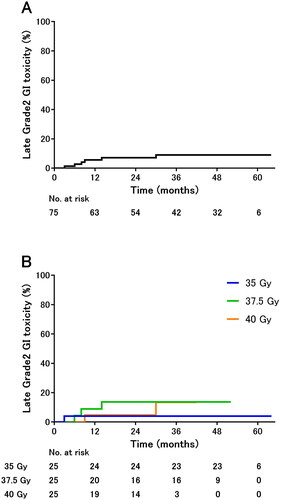
The 2-year occurrence rates of grade 2 GU toxicities were 34% in all cohorts () and 21%, 40%, and 42% in the 35-, 37.5-, and 40-Gy cohorts, respectively (). The occurrence risk of grade 2 GU toxicities increased significantly with dose escalation (p = 0.0256). Even under adjustment for PTV, the lower 95% confidence intervals for the adjusted HRs of 37.5 and 40 were above 1 when the dose of 35 Gy was used as a reference. The adjusted p-values for the test results under adjustment for multiplicity by Dunnett’s test were 0.050 for 37.5 vs. 35 Gy and 0.044 for 40 vs. 35 Gy (Supplementary Table).
Figure 1. Cumulative incidence of late grade 2 genitourinary toxicities in (A) all cohorts and (B) each cohort.
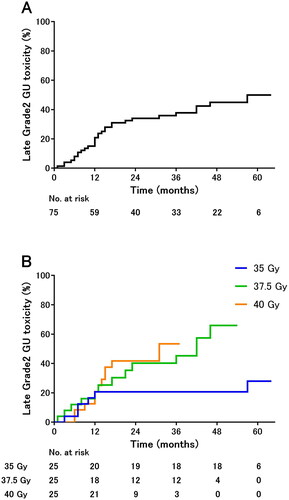
The 2-year occurrence rate of grade 2 GI toxicities was 7% in all cohorts () and 4%, 14%, and 5% in the 35-, 37.5-, and 40-Gy cohorts, respectively (). The difference was not significant.
Discussion
In this study, we determined the correlation between RT dose and risk of toxicities. Generally, patients with localised PCa have a good prognosis and long life expectancy. Therefore, the risk of adverse events, especially late adverse events, should be minimised to improve patients’ quality of life. In two previously reported randomised phase III trials, the occurrence rates of grade ≥ 2 acute GU and GI toxicity after SBRT were approximately 20–30% and 5–10%, respectively, which were similar to or slightly higher than those after conventional fractionated RT [Citation14,Citation15]. The results from this study were comparable to the results obtained in previous studies, and the occurrence rates of GI and GU acute toxicities were similar among the 3 cohorts.
In contrast to acute toxicities, the cumulative rates of late toxicities, especially GU toxicities, increased as the dose of RT increased. Although PTV differences existed among the three cohorts, the SBRT dose had a greater impact on toxicity than PTV. The 5-year occurrence rates of grade 2 GU and GI toxicities in a large-scale pooled analysis of the cohort studies (n = 2142 patients) were 11.2% and 4.5%, respectively [Citation16]. In another phase III trial, the 2-year occurrence rates of grade ≥2 GU and GI toxicities after SBRT were 9% and 6%, respectively, and were similar to the rates obtained after conventional fractionated RT [Citation14]. In our study, the occurrence rate of GI toxicities was comparable and acceptable in the 3 dose cohorts, whereas the occurrence rate of grade 2 toxicities was slightly higher in the 37.5-Gy cohort than in the 35- and 40-Gy cohorts. Furthermore, a polyethylene glycol hydrogel spacer has been developed to displace the rectum away from the prostate, and its validity to minimise rectal radiation injury has been recognised [Citation17,Citation18]. Therefore, treatment with SBRT, using the appropriate method and materials, can be considered tolerable for the GI tract. Conversely, the 2-year occurrence rate of grade 2 GU toxicities observed in this study was higher than that in the past reports. In particular, the risk was non-negligible in the 37.5- and 40-Gy cohorts (40% and 42%, respectively). High-dose SBRT is a burden on patients. Previous studies evaluating the correlation between RT dose and GU toxicities have reported contradictory results. Musunuru et al. [Citation19] reported the comparative outcomes of two SBRT studies in which doses of 35 and 40 Gy per 5 fractions were used. The occurrence rates of grade 1 and 2 GU toxicities were significantly higher in patients who received 40-Gy SBRT than in those who received other doses and were similar to those reported in our study. Conversely, Zelefsky et al. [Citation20] reported no significant differences in rates of toxicities among the dose groups in their dose escalation study. In our study, the positive correlation between toxicities and dose escalation was possibly due to the consequent increase in dose distributed to the OAR. When calculating the BED of normal tissues, the α/β ratio is usually set at approximately 3. Using this value, the BED of 37.5 Gy per 5 fractions is 131.25 Gy, which is equivalent to 78.75 Gy in 2-Gy fraction. The 2-year occurrence rate of late grade ≥ 2 GU toxicities was approximately 10%, referring to the previous reports that used conventional fractionated RT dose of approximately 75–80 Gy [Citation14,Citation21]. In contrast, the corresponding data for the 37.5-Gy cohort in our study were higher than those for the 35-Gy cohort. Alayed et al. [Citation22] reported that the dose-volume parameters of the bladder (mean dose and volume irradiated dose of 38 Gy) were significant predictors of GU toxicities after SBRT for PCa. Some SBRT trials specify dose constraints to high-dose areas in the bladder and urethra [Citation7,Citation20], which we did not specify.
Thus, the dose constraints of the bladder and urethra should be established apart from those of conventional fractionated RT when considering SBRT for PCa. Although a recent practice guideline stated that SBRT can be applied to high-risk cases and higher dose-fractionation techniques, such as 40 Gy per 5 fractions, have been proposed as examples [Citation13], in view of our findings, stereotactic radiotherapy with high doses may require careful attention to toxicity. In addition, some authors reported that the risk reduction of toxicities could be achieved via RT with a certain period between fractions [Citation23,Citation24]. The method of SBRT without close intervals may be beneficial, but the risk of RT effect attenuation should be carefully minimised.
Herein, we evaluated the short-term efficacy of SBRT for low- or intermediate-risk PCa. Only 2 patients (3% of all cohorts) experienced clinical recurrence, of which 1 had lymph node and distant (bone) metastases without local recurrence. Although the short-term results of our treatment are favourable, the follow-up period was too short to definitively conclude the efficacy of SBRT since PCa relapse occasionally becomes evident after 5 years of latency. Previous findings should therefore be considered when discussing the efficacy of SBRT. For instance, a systematic review and meta-analysis of prospective studies reported that the 7-year bRF survival rate of patients who underwent SBRT for localised PCa was satisfactory (93.7%) [Citation25]. Referring to studies using the tumour control probability model, a 5-year bRF rate approaching 95% is possible with 35 Gy per 5 fractions for low- or intermediate-risk prostate cancer [Citation26]. Alayed et al. [Citation27] reported a favourable bRF rate after SBRT of 35 Gy per 5 fractions, which was not inferior to 40 Gy per 5 fractions. In contrast, previous studies also reported a difference in antitumor effect based on the dose. Zelefsky et al. [Citation20] reported residual cancer cells in patients who did not experience PSA failure, with a rate of positive findings in post-treatment biopsy of >15% after SBRT of 32.5–37.5 Gy per 5 fractions, whereas the corresponding value for 40 Gy was 7.7%. In addition, PSA decline is more significant in patients who received SBRT of 40 Gy than in those received 35 Gy, while SBRT of 40 Gy potentially is beneficial in terms of therapeutic effects [Citation27,Citation28]. We believe that the treatment outcomes achieved with SBRT of 35 or 37.5 Gy per 5 fractions in this study are satisfactory. However, considering previous findings, tumour recurrence in the 35-Gy or 37.5-Gy cohorts may occur with a longer follow-up period. Although an SBRT dose of 35–36.25 Gy per 5 fractions for low- or intermediate-risk PCa has been used in clinical practice, whether the SBRT efficacy is dose-dependent remains to be determined considering the long-term outcomes of our and other studies.
A major limitation of our study was the short follow-up time. The cumulative rate of toxicities after high-dose RT for PCa gradually increases with a long follow-up period. GU toxicities have been reported even after 10 years of treatment [Citation21]. Our median follow-up time of 48 months was very short, not only from the perspective of evaluating efficacy but also from the risk of late toxicities. Therefore, long-term analysis is required to evaluate cancer recurrence and late toxicities. Another limitation was the non-randomised design of this study and the small number of patients enrolled. Although there were no significant differences in patients’ characteristics, we could not rule out potential treatment bias while comparing the 3 cohorts. Despite these limitations, our results regarding the increased risk of late toxicities with increasing RT dose were significant and clinically meaningful.
Dose escalation of SBRT increased the risk of late GU toxicities in PCa; late grade 2 GU toxicities were positively correlated with dose escalation of SBRT. Therefore, an SBRT dose of 35 Gy per 5 fractions is safe in patients with low- or intermediate-risk PCa; however, longer follow-up is required to confirm our findings. Higher doses of SBRT should be applied with caution.
Ethics statement
The study protocol was approved by the Human Ethics Review Committee of the Osaka University Hospital and signed informed consent form was obtained from all patients.
Supplemental Material
Download MS Word (25.8 KB)Disclosure statement
The authors report there are no competing interests to declare.
Data availability statement
The datasets analysed in the current study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Global burden of disease cancer collaboration. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-Adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol. 2019;5(12):1749–1768.
- Dasu A, Toma-Dasu I. Prostate alpha/beta revisited-an analysis of clinical results from 14 168 patients. Acta Oncol. 2012;51(8):963–974.
- Morgan SC, Hoffman K, Loblaw DA, et al. Hypofractionated radiation therapy for localized prostate cancer: an ASTRO, ASCO, and AUA evidence-based guideline. J Clin Oncol. 2018;36(34):JCO1801097.
- Mottet N, van den Bergh RCN, Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer-2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79(2):243–262.
- King CR, Freeman D, Kaplan I, et al. Stereotactic body radiotherapy for localized prostate cancer: pooled analysis from a multi-institutional consortium of prospective phase II trials. Radiother Oncol. 2013;109(2):217–221.
- Stone NN, Potters L, Davis BJ, et al. Customized dose prescription for permanent prostate brachytherapy: insights from a multicenter analysis of dosimetry outcomes. Int J Radiat Oncol Biol Phys. 2007;69(5):1472–1477.
- Draulans C, van der Heide UA, Haustermans K, et al. Primary endpoint analysis of the multicentre phase II hypo-FLAME trial for intermediate and high risk prostate cancer. Radiother Oncol. 2020;147:92–98.
- Hannan R, Tumati V, Xie XJ, et al. Stereotactic body radiation therapy for low and intermediate risk prostate cancer-results from a multi-institutional clinical trial. Eur J Cancer. 2016;59:142–151.
- National Cancer Institute [Internet]. Common terminology criteria for adverse events v4.0. Bethesda (MD): NIH; 2010. [cited 2022 July 25]. Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.
- Roach M, Hanks G, Thames H, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO phoenix consensus conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965–974.
- Sobin LH, Gospodarowics MK, Wittekind CH. TNM classification of malignant tumors. 7th ed. Hoboken, NJ: Wiley-Blackwell; 2009.
- Barry MJ, Fowler FJ, Jr O'Leary MP, et al. The American urological association symptom index for benign prostatic hyperplasia. The measurement committee of the American urological association. J Urol. 1992;148(5):1549–1557; discussion 1564.
- National Comprehensive Cancer Network [Internet]. Clinical practice guidelines in oncology. Prostate cancer. Plymouth Meeting (PA): NCCN; [cited 2022 July 25]. Available from: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1459.
- Widmark A, Gunnlaugsson A, Beckman L, et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet. 2019;394(10196):385–395.
- Brand DH, Tree AC, Ostler P, et al. Intensity-modulated fractionated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): acute toxicity findings from an international, randomised, open-label, phase 3, non-inferiority trial. Lancet Oncol. 2019;20(11):1531–1543.
- Kishan AU, Dang A, Katz AJ, et al. Long-term outcomes of stereotactic body radiotherapy for low-risk and intermediate-risk prostate cancer. JAMA Netw Open. 2019;2(2):e188006.
- Hamstra DA, Mariados N, Sylvester J, et al. Continued benefit to rectal separation for prostate radiation therapy: final results of a phase III trial. Int J Radiat Oncol Biol Phys. 2017;97(5):976–985.
- Karsh LI, Gross ET, Pieczonka CM, et al. Absorbable hydrogel spacer use in prostate radiotherapy: a comprehensive review of phase 3 clinical trial published data. Urology. 2018;115:39–44.
- Musunuru HB, Quon H, Davidson M, et al. Dose-escalation of five-fraction SABR in prostate cancer: toxicity comparison of two prospective trials. Radiother Oncol. 2016;118(1):112–117.
- Zelefsky MJ, Kollmeier M, McBride S, et al. Five-year outcomes of a phase 1 dose-escalation study using stereotactic body radiosurgery for patients with low-risk and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2019;104(1):42–49.
- Zelefsky MJ, Levin EJ, Hunt M, et al. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70(4):1124–1129.
- Alayed Y, Davidson M, Quon H, et al. Dosimetric predictors of toxicity and quality of life following prostate stereotactic ablative radiotherapy. Radiother Oncol. 2020;144:135–140.
- Zimmermann M, Taussky D, Menkarios C, et al. Prospective phase II trial of once-weekly hypofractionated radiation therapy for low-risk adenocarcinoma of the prostate: late toxicities and outcomes. Clin Oncol (R Coll Radiol). 2016;28(6):386–392.
- Quon HC, Ong A, Cheung P, et al. Once-weekly versus every-other-day stereotactic body radiotherapy in patients with prostate cancer (PATRIOT): a phase 2 randomized trial. Radiother Oncol. 2018;127(2):206–212.
- Jackson WC, Silva J, Hartman HE, et al. Stereotactic body radiation therapy for localized prostate cancer: a systematic review and meta-analysis of over 6,000 patients treated on prospective studies. Int J Radiat Oncol Biol Phys. 2019;104(4):778–789.
- Royce TJ, Mavroidis P, Wang K, et al. Tumor control probability modeling and systematic review of the literature of stereotactic body radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2021;110(1):227–236.
- Alayed Y, Cheung P, Pang G, et al. Dose escalation for prostate stereotactic ablative radiotherapy (SABR): late outcomes from two prospective clinical trials. Radiother Oncol. 2018;127(2):213–218.
- Helou J, D'Alimonte L, Quon H, et al. Stereotactic ablative radiotherapy in the treatment of low and intermediate risk prostate cancer: is there an optimal dose? Radiother Oncol. 2017;123(3):478–482.