Abstract
Objectives
According to the CONCORD-3 study, the 5-year survival rate of lung cancer patients in Finland has not improved during the twenty-first century. In the present study, we evaluated the survival trends of lung cancer patients diagnosed and treated in one of the five university hospitals in Finland to determine possible explanatory factors behind the lack of improved survival.
Material and methods
This retrospective population-based study included all lung cancer patients diagnosed in Tampere University Hospital in 2007–2019 (N = 3041). The study population was divided into two subcohorts: the patients diagnosed in 2007–2012 and those diagnosed in 2013–2019. The two subcohorts were then compared to analyze the temporal changes in survival and the distribution of prognostic factors.
Results
A comparison of the patients diagnosed in 2007–2012 and 2013–2019 showed that the patients’ overall survival had remained unchanged. The median overall survival was 8.7 months in the earlier subcohort and 9.2 months in the later subcohort. The respective 5-year survival rates were 16.6% and 17.8%, and these differences were not statistically significant. The proportion of stage IV patients (approximately 59% in both subcohorts) and their risk of death were similar for the two subcohorts. According to the regression analysis, male gender, advanced stage, and poor Eastern Cooperative Oncology Group performance status were independent risk factors for death, while a never-smoking status and mutation-positive disease were associated with a decreased risk of death, but only in the later cohort.
Conclusion
Echoing the results of CONCORD-3, this study confirmed that the real-world survival of unselected lung cancer populations in Finland has not improved over the last 15 years, mainly because of the unchanged proportions of patients with late-stage lung cancer. This calls for earlier recognition of lung cancer, achieved by screening and increasing awareness of the disease.
Introduction
Lung cancer is the leading cause of cancer-related deaths globally, accounting for 1.8 million deaths worldwide in 2020 [Citation1] and 2358 deaths in Finland in 2019 [Citation2]. Although tobacco smoking among Finnish citizens, especially men, has decreased markedly since the 1970s [Citation3], the number of new lung cancer cases is projected to increase at least until the year 2035 owing to the aging population [Citation2]. Over the last decade, multiple new treatment options for metastatic non-small-cell lung cancer (NSCLC) have emerged, such as tyrosine kinase inhibitors (TKIs) for patients with activating mutations or rearrangements in certain genes and immune checkpoint inhibitors (ICIs). These treatments have improved survival rates in certain subsets of lung cancer patients [Citation4–6].
CONCORD is a large-scale global study that evaluates changes in the survival of 18 types of cancer patients across various countries and territories based on the data available in national or regional cancer registries. In the third cycle of this study, CONCORD-3, the survival rates of patients diagnosed between 2000 and 2014 were reported [Citation7]. The study revealed that in Finland, the age-standardized 5-year net survival of lung cancer patients had improved only marginally from 11.9% to 13.0%. In contrast, the 5-year survival in other Northern European countries, for example, Sweden, Norway, Iceland, and Estonia, had increased more markedly from 13.9% to 19.5%, 12.3% to 19.0%, 14.1% to 20.2%, and 10.8% to 16.9%, respectively. The most remarkable increase in the 5-year survival rate, namely, from 9.5% to 16.6%, among Nordic lung cancer patients was recorded in Denmark. Outside Northern Europe, the 5-year survival rates in the UK and the US increased from 8.3% to 13.3% and from 17.0% to 21.2%, respectively.
The patients included in CONCORD-3 were diagnosed and treated before the widespread use of TKIs and ICIs. The improved survival rates could therefore likely be ascribed to earlier detection, which facilitates curative treatment. For example, in Norway, an increase in the proportion of early stage disease and the use of therapies with curative intent, such as surgical resection and stereotactic body radiotherapy (SBRT), were noted alongside improved survival [Citation8]. According to the Norwegian retrospective national cancer registry-based study, 38% of patients were treated with curative intent in 2016 compared to 23% in 2001. Meanwhile, the proportion of localized disease increased significantly from 11% to 20% in women and from 13% to 16% in men. Furthermore, the 5-year survival rates of all lung cancer patients doubled from 9% to 20%. A similar shift toward earlier diagnosis was also demonstrated in the UK. A single-center retrospective study found that in 2010–2017, the proportion of stage I patients among all NSCLC patients increased from 16% to 28%, while that of stage IV patients decreased from 57% to 39% [Citation9].
Similar real-world data on the survival rates of lung cancer patients in Finland are scarce. The existing studies are either dated [Citation10] or focus solely on surgical outcomes [Citation11,Citation12]. Accordingly, the present retrospective descriptive population-based cohort study aimed to evaluate current trends in the incidence and mortality of all lung cancer patients diagnosed and treated in one Finnish university hospital and to determine the prognostic factors that may explain the changes in survival over time.
Material and methods
Patients
Tampere University Hospital (TAUH) provides secondary health care services to the approximately 520,000 inhabitants of the Pirkanmaa region in Finland. All lung cancer patients from this region are diagnosed and treated at TAUH. In our study, all lung cancer patients diagnosed at TAUH between 1 January 2007 and 31 December 2019 were included. The subjects were identified from the hospital’s electronic medical record system (eMRS) by following the World Health Organization’s International Statistical Classification of Diseases and Related Health Problems: 10th Revision (ICD-10) code for lung and bronchial cancer, C34. The case files were manually reviewed to exclude patients with a malignant tumor in the lung but not primary lung cancer. We were thus able to capture both pathologically and clinically diagnosed cases of lung cancer.
The variables extracted from the eMRS included the day of diagnosis; age at diagnosis; gender; day of death; comorbidities; types and dates of therapeutic interventions (surgery, radiotherapy, and anti-cancer drug therapy); histology or cytology; tumor, node, metastasis (TNM) classification; stage; Eastern Cooperative Oncology Group (ECOG) performance status; mutation status of the EGFR and ALK genes; and smoking status with pack-years smoked when applicable. The patients’ comorbidities were captured via the ICD-10 codes, which were found in each patient’s case history preceding the day of their lung cancer diagnosis. The comorbidities were weighted and grouped according to the Charlson Comorbidity Index (CCI) [Citation13]. Therapeutic interventions were identified using the national procedure codes [Citation14], which specify the technique and extent of any surgeries, intent and target of radiotherapy, and goal and type of anti-cancer drug therapy delivered. SBRT and conventionally fractionated definitive radiotherapy, as well as concurrent chemoradiotherapy, were grouped together under the term ‘radiotherapy with curative intent.’ The data concerning tumor histology, TNM classification, stage of disease, mutation status, ECOG performance status, and smoking status were not structurally entered in the hospital’s eMRS. Consequently, we conducted eMRS searches using the keywords, phrases, and character strings associated with the aforementioned variables and reviewed and classified the results manually for each case. The TNM Classification of Malignant Tumours, sixth edition [Citation15], was used for the TNM classification and stage grouping of the patients diagnosed before 2010, the seventh edition [Citation16] was utilized for those diagnosed between 2010 and 2016, and the eighth edition [Citation17] for those diagnosed in and after 2017. The patient performance status was set as the ECOG value closest to their day of diagnosis.
Statistical analyses
For crude incidence calculations, we obtained the annual population numbers of Pirkanmaa region from Official Statistics of Finland [Citation18]. The annual crude incidence rates were expressed as the number of new lung cancer cases per 100,000 persons. Survival was calculated based on the number of days between the day of lung cancer diagnosis and the day of death. Right-censoring occurred when the patients were followed up for a maximum of five years, or until the cutoff date for follow-up (i.e., 30 April 2020). The median overall survival and 1- and 5-year survival probabilities were estimated using the Kaplan–Meier method. The differences between the two subcohorts were evaluated in terms of the baseline demographics, prognostic factors, treatments administered, and survival. We assessed the effects of several confounding factors (listed in ) on 5-year survival using multivariable-adjusted Cox proportional hazards regression analysis. The assumptions for proportional hazards were tested. The national procedure codes for radiotherapy and anti-cancer drug therapy were not routinely available in the TAUH eMRS prior to January 2013, and for this reason, the patients diagnosed before 2013 were not formally included in the analyses pertaining to these treatment modalities. Seven patients were excluded from the survival analysis because their lung cancer diagnoses were based only on death certificate data, and they therefore had negative survival times. The statistical significance of the difference between the two subcohorts was calculated via the Mann–Whitney U-test, chi-squared test, Fisher’s exact test, and log-rank test, where appropriate. Two-sided p values of less than .05 were considered significant. The statistical analyses were performed using IBM’s SPSS Statistics version 28 (Armonk, NY) for macOS and RStudio version 2022.12.0 + 353 for macOS (we ran R Statistical Software version 4.2.2 with the survival, survminer, and tidyverse packages, R Foundation for Statistical Computing, Vienna, Austria).
Table 1. Comparison between the cohorts in terms of the distribution of the prognostic factors of lung cancer (N = 3041).
This study was approved by the administration of TAUH (research permit number R20122/2020).
Results
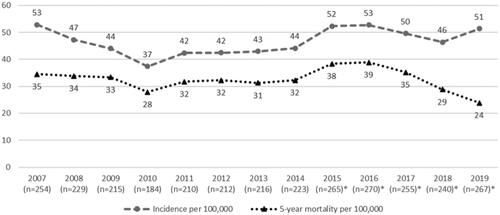
We identified 4472 individual patients with the diagnosis code C34 recorded in the TAUH eMRS during the study period. Among them, 1348 patients were citizens of regions other than Pirkanmaa, and they were excluded from this study. A further 83 patients were identified as having pulmonary metastasis from a primary tumor outside of the lung and had been misclassified as C34, so they, too, were excluded. The final study cohort comprised 3041 eligible lung cancer patients. For comparison purposes, we divided the patients into two subcohorts: those whose lung cancer was diagnosed in 2007–2012 (‘the earlier subcohort’) and those diagnosed in 2013–2019 (‘the later subcohort’). This allowed sufficient follow-up time for the patients in the later subcohort. The earlier and later subcohorts comprised 1304 and 1737 patients, respectively. The number of new lung cancer cases per year varied from 184 in 2010 to 270 in 2016. In the year 2007, the population of Pirkanmaa was 480,634, while in 2019, it was 519,872. The annual incidence rates of lung cancer increased slightly with an average of 44.4 new lung cancer cases per 100,000 persons in 2007–2012 compared to 48.5 new cases per 100,000 in 2013–2019 ().
Figure 1. Annual incidence and all-cause 5-year mortality rates (per 100,000) of lung cancer. n = annual number of new lung cancer patients. *Follow-up time is less than 5 years.
Prognostic factors and treatments
The ratios of the different stages of the disease remained unchanged during the study period, with the stages I and II patients accounting for 21.4% and 23.0% of the earlier and later subcohorts and the stage IV patients comprising 57.8% and 59.5% of these subcohorts, respectively (p = .21) (). The stage distribution of the incident lung cancer cases varied from year to year, but a clear shift toward an earlier stage at diagnosis was not evident (Supplementary Figure 1). The median age at lung cancer diagnosis was 70.4 years in the earlier subcohort, and it increased modestly but significantly to 71.4 years in the later subcohort. The proportion of females increased borderline significantly from 32.7% to 36.2% between the earlier and later subcohorts (p = .045). The proportion of patients with adenocarcinoma histology increased markedly from 32.1% to 41.1% between the respective subcohort study periods (p < .001), while that of the squamous cell carcinoma patients decreased from 23.8% to 21.9%. The proportion of small cell carcinoma patients remained almost unchanged at 12.4% in the earlier subcohort and 13.4% in the later subcohort, and the incidence of mutation-positive disease among all the lung cancer patients increased from 1.5% to 3.9%, which corresponded to 4.5–9.4% of the adenocarcinoma patients. The number of patients with high (three or more) CCI values and poor performance status (ECOG 3–4) was significantly higher in the later subcohort than in the earlier subcohort. Finally, no changes were apparent in smoking habits over time, and the mean number of pack-years smoked was 40 in both subcohorts. Approximately, 70% of the never-smokers were female (data not presented).
The proportion of patients who were surgically resected increased significantly from 14.1% in the earlier subcohort to 18.9% in the later subcohort (p < .001) (Supplementary Table 1). The number of patients who received ICIs or TKIs was low in both subcohorts. However, the proportion of patients who received ICIs and TKIs increased from 0.2% to 1.7% and from 1.5% to 4.2% between the earlier and later subcohorts, respectively. Among the patients diagnosed in 2013–2019, 11% received radiotherapy with curative intent, 26% had palliative radiotherapy, and 33% underwent palliative chemotherapy, while 25% of the patients did not receive any anti-cancer therapy.
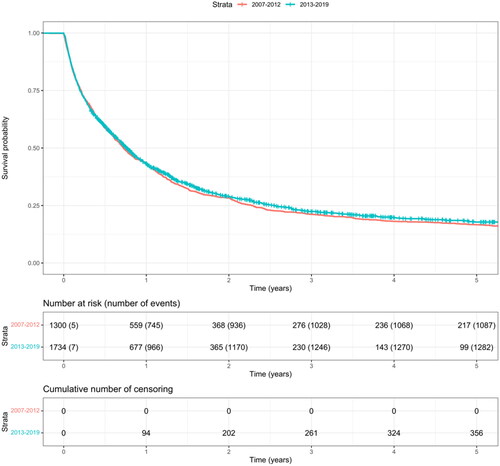
Survival
The annual 5-year all-cause mortality rates have remained constant over time (). The median overall survival was similar in both subcohorts (). The median survival was 264 days (8.7 months) in the earlier subcohort and 280 days (9.2 months) in the later subcohort (p = .26). The estimated 1- and 5-year overall survival rates were, respectively, 42.9% and 16.6%, in the earlier subcohort and 43.3% and 17.8%, in the later subcohort.
shows the results of the multivariate Cox regression analysis for survival. In both subcohorts in the model, increasing age, male gender, advanced stage, small cell histology, a CCI of 5 or greater, and a worsening ECOG performance status were independent factors for an increased risk of death. Additionally, among the patients diagnosed in 2007–2012, an unknown smoking status was a significant risk factor. On the other hand, carcinoid histology, a never-smoking status, mutation-positive disease, and clinical diagnosis of lung cancer were significantly associated with a decreased risk of death among the patients diagnosed in 2013–2019, while carcinoid histology was the only significant protective risk factor among the patients in the earlier subcohort.
Table 2. The effects of prognostic factors on the risk of death (all causes): results of the multivariable-adjusted Cox regression analysis.
There was no change in survival for any type of treatment modality between the two cohorts (Supplementary Table 2). The longest median survival times (i.e., 2253 days (74.0 months)) were recorded among the surgically treated patients in the earlier cohort, while the median value was not reached in the later cohort. Conversely, when no anti-cancer therapy was administered, the median survival was only 90 days in the earlier cohort and 44 days in the later cohort. The estimated 1- and 5-year survival rates of the surgically resected patients were, respectively, 87.5% and 56.0% in the earlier subcohort and 89.7% and 60.9% in the later subcohort.
Discussion
The results of our population-based study indicated that the real-world survival of lung cancer patients diagnosed and treated in one public health organization in Finland did not increase during the period 2007–2019. Moreover, a significantly larger proportion of patients underwent surgery in the later subcohort than the earlier subcohort, and the survival of the operated patients was excellent in both subcohorts. However, this did not translate into improved prognoses across the entire study population, most likely because of the overwhelmingly large and unchanged percentage of stage IV patients, whose survival was poor in most cases. It has previously been demonstrated that disease stage is the most important prognostic factor in lung cancer patients, with stage IV patients having an almost eightfold higher risk of death compared to stage I patients [Citation19]. When compared to the stage I patients in our study, the risk of death among the stage IV patients was almost sixfold in the earlier subcohort and 11-fold in the later subcohort. Furthermore, our data indicated that the average age of the lung cancer patients has significantly increased over the study period. Patients’ performance status at diagnosis has become poorer, and they had greater numbers of comorbidities. This shift may counteract the positive changes in favorable prognostic factors observed in our study, such as the increased proportion of females and patients with adenocarcinoma. Notably, approximately one-fourth of the patients in the later subcohort in our study did not receive any anti-cancer therapy. This mirrors the proportion (28%) of ECOG 3–4 patients, who, according to the European lung cancer treatment guidelines [Citation20], should be offered the best supportive care instead of systemic cancer therapy.
The main finding of this study is consistent with that of CONCORD-3, which indicated that the 5-year survival rate of lung cancer patients has remained unchanged in Finland during the first two decades of the twenty-first century. This, however, contrasts with the improved survival recorded in nearby countries with similar social structures and healthcare systems. Nevertheless, the 5-year overall survival rates of the present study population (17% in the earlier subcohort and 18% in the later subcohort) are clearly superior to the values reported in CONCORD-3, and they match the rates recorded in other Nordic countries. The reason behind the discrepancy between the two studies is unclear. However, Finland has remarkable interregional imbalances in cancer mortality, and the Pirkanmaa region has lower-than-average lung cancer mortality among both men and women [Citation2]. A nationwide registry of Finnish lung cancer patients would help shed more light on the survival differences between regions and the associated contributing factors. Apart from CONCORD-3, there is a paucity of real-world survival data related to all lung cancer histology types. Studies that exclusively explored NSCLC patient populations were recently published [Citation9,Citation21,Citation22], and the patient characteristics and survival in the UK and Scandinavian study results were similar to those of our study population.
As previously mentioned, the improved survival of Norwegian lung cancer patients has been attributed to the increased proportion of early stage disease, which provides more opportunities for the administration of curative treatments. One approach to promote early diagnosis is the implementation of lung cancer screening programs. The results of two large prospective studies [Citation23,Citation24] showed that screening with low-dose computed tomography decreased lung cancer-specific mortality in high-risk populations. Following the publication of these results, several countries started screening programs, but data on the real-world effects of screening are lacking. Although a position paper about the implementation of lung cancer screening in Nordic countries has been published [Citation25], and several screening trials are underway in neighboring countries, Finland is yet to start any lung cancer screening initiatives.
Treatment advances in metastatic lung cancer, such as ICIs and TKIs for mutation-positive disease, were not yet fully in use at the time our data were collected, especially among the patients in the earlier cohort. As more drugs have become available, testing for targetable mutations has become systematic, and the incidence of mutation-positive disease has increased. Encouraging signs of survival advantage with targeted therapies have already been observed: in our study, the median survival of the patients diagnosed in 2013–2019 who received ICIs or TKIs was 1011 days (33 months) and 659 days (22 months), respectively. A systematic review on the survival of never-smokers indicated that the patients treated with TKIs had a high 5-year survival rate of up to 63% [Citation26]. Similarly, the significantly decreased risk of death among never-smokers in the later subcohort of our study population may be attributable to better recognition of mutation-positive diseases with the routine use of molecular pathology diagnostics and the utilization of TKIs. The use of ICIs has become more widespread, especially among the patients diagnosed in the later years of the 2013–2019 subcohort, but the relatively short follow-up time of these patients may contribute to the apparent lack of improved survival among stage IV patients.
The main weakness of this study was the incompleteness of the source data, which largely had to be gathered from an unstructured text pool of case history entries. We were thus unable to locate information about disease stage for 17% of the patients included in this study, histologic subtype for 12% of the patients, and ECOG performance status for 27%. This may have led to a bias toward diseases with a worse prognosis because when the exact TNM classification or stage information was unavailable, we performed case history searchers using specific key words. It was then easier to recognize patients with metastatic disease than those with limited disease. It is also likely that the original entry of the ICD-10 codes for comorbidities was inadequate. This may have led to an overestimation of the number of CCI 0 patients, as suggested by the paradoxical finding that their risk of death was higher than that of CCI 1–2 patients. Finally, the national procedure codes for radiotherapy and anti-cancer drug therapy were not available in the TAUH eMRS until 2013. The number of patients in the earlier subcohort known to have received anti-cancer therapy was therefore very low, and we could not perform formal comparisons between the subcohorts pertaining to therapies other than surgery.
Notwithstanding, this study had several strengths. First, we used an unselected population of all lung cancer patients who were diagnosed over a period spanning almost 15 years. This helped us obtain insights into the real-world survival, treatment trends, and characteristics of the lung cancer patients diagnosed at TAUH over the study period. Clinical trials enroll extremely niche patient populations, which do not fully represent the patients encountered in daily practice. Our subject identification strategy also enabled us to include patients who were clinically diagnosed without histological or cytological confirmation of the disease; such patients are not usually captured in predominantly pathology-based registries, such as the Finnish Cancer Registry. Moreover, we were able to gather a broad spectrum of data on the patients’ characteristics, including comorbidities, smoking habits, and the therapies delivered to the patients.
Lung cancer remains a disease with a poor prognosis, especially if it is diagnosed in its later stages. Efforts must be made to promote earlier diagnoses, which would allow for the delivery of treatments with curative intent and increase survival at a population level. This could be achieved by increasing awareness about the symptoms of lung tumors at community and primary healthcare personnel levels and implementing national lung cancer screening programs. Furthermore, comprehensive, dedicated registries of lung cancer patients should be established to facilitate the benchmarking and quality assurance of diagnostic pathways and treatments.
Author contributions
Jarkko Ahvonen: conceptualization, methodology, formal analysis, investigation, data curation, writing of the original draft, visualization, and funding acquisition. Tiina Luukkaala: methodology, formal analysis, and writing–preview and editing. Tarja Laitinen: conceptualization, methodology, resources, writing–preview and editing, supervision, project administration, and funding acquisition. Arja Jukkola: conceptualization, methodology, resources, writing–preview and editing, supervision, project administration, and funding acquisition.
Supplemental Material
Download MS Word (19 KB)Supplemental Material
Download EPS Image (14.8 KB)Supplemental Material
Download MS Word (22.6 KB)Acknowledgements
The authors thank the Clinical Informatics Unit at Tampere University Hospital for their support with the data curation, visualization, and analysis.
Disclosure statement
Jarkko Ahvonen, Tarja Laitinen, and Arja Jukkola have received funding (grants) from Tampere University Hospital. Tiina Luukkaala has no conflict of interest to declare.
Data availability statement
The data that support the findings of this study are available from the corresponding author (JA) upon reasonable request.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249.
- Pitkäniemi J, Malila N, Tanskanen T, et al. Cancer in Finland 2019. Cancer society of Finland publication no. 98. Helsinki: Cancer Society of Finland; 2021.
- Ruokolainen O, Heloma A, Jousilahti P, et al. Thirty-eight-year trends of educational differences in smoking in Finland. Int J Public Health. 2019;64(6):853–860.
- Memmott RM, Wolfe AR, Carbone DP, et al. Predictors of response, progression-free survival, and overall survival in patients with lung cancer treated with immune checkpoint inhibitors. J Thorac Oncol. 2021;16(7):1086–1098.
- Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382(1):41–50.
- Britschgi C, Addeo A, Rechsteiner M, et al. Real-world treatment patterns and survival outcome in advanced anaplastic lymphoma kinase (ALK) rearranged non-small-cell lung cancer patients. Front Oncol. 2020;10:1299.
- Allemani C, Matsuda T, di Carlo V, et al. Global surveillance of trends in cancer survival 2000–2014 (CONCORD-3): analysis of individual records for 37,513,025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–1075.
- Solberg S, Nilssen Y, Brustugun OT, et al. Increase in curative treatment and survival of lung cancer in Norway 2001–2016. Eur J Epidemiol. 2019;34(10):951–955.
- Snee M, Cheeseman S, Thompson M, et al. Treatment patterns and survival outcomes for patients with non-small cell lung cancer in the UK in the preimmunology era: a REAL-Oncology Database Analysis from the I-O optimise initiative. BMJ Open. 2021;11(9):e046396.
- Mäkitaro R, Pääkko P, Huhti E, et al. Prospective population-based study on the survival of patients with lung cancer. Eur Respir J. 2002;19(6):1087–1092.
- Gunn J, Valo J, Sipilä J, et al. Trends and results of lung cancer surgery in Finland between 2004 and 2014. Eur J Cardiothorac Surg. 2018;54(1):127–133.
- Helminen O, Valo J, Andersen H, et al. Extended resections and other special cases in lung cancer surgery: real-world population-based outcomes. Thorac Cancer. 2020;11(10):2932–2940.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Lehtonen J, Lehtovirta J, Mäkelä-Bengs P. THL-Toimenpideluokitus—THL-Åtgärdskalssifikation. Helsinki (Finland): THL; 2013.
- Sobin L, Wittekind C. TNM classification of malignant tumours. 6th ed. New York: Wiley-Liss; 2002.
- Sobin L, Gospodarowicz M, Wittekind C. TNM classification of malignant tumours. 7th ed. Chichester (UK): John Wiley & Sons, Ltd; 2009.
- Brierley J, Gospodarowicz M, Wittekind C. TNM classification of malignant tumours. 8th ed. Chichester (UK): John Wiley & Sons, Ltd; 2017.
- Official Statistics of Finland. Population structure (e-publication). Helsinki: Statistics Finland; 2023.
- Alexander M, Wolfe R, Ball D, et al. Lung cancer prognostic index: a risk score to predict overall survival after the diagnosis of non-small-cell lung cancer. Br J Cancer. 2017;117(5):744–751.
- Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl. 4):iv192–iv237.
- Ekman S, Horvat P, Rosenlund M, et al. Epidemiology and survival outcomes for patients with NSCLC in Scandinavia in the preimmunotherapy era: a SCAN-LEAF retrospective analysis from the I-O optimise initiative. JTO Clin Res Rep. 2021;2(5):100165.
- Aarts MJ, van den Borne BE, Biesma B, et al. Improvement in population-based survival of stage IV NSCLC due to increased use of chemotherapy. Int J Cancer. 2015;136(5):E387–E395.
- National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409.
- De Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382(6):503–513.
- Pedersen JH, Sorensen JB, Saghir Z, et al. Implementation of lung cancer CT screening in the Nordic countries. Acta Oncol. 2017;56(10):1249–1257.
- Casal-Mouriño A, Valdés L, Barros-Dios JM, et al. Lung cancer survival among never smokers. Cancer Lett. 2019;451:142–149.